Abstract
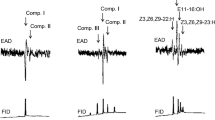
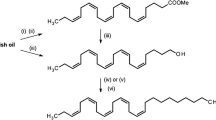
Close-range sexual communication of the egg parasitoid wasp Ooencyrtus kuvanae (Hymenoptera: Encyrtidae) takes place on host gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae), egg masses. We tested the hypothesis that mate recognition in O. kuvanae is mediated, in part, by low-volatility cuticular hydrocarbon (CHC) pheromone components. Gas chromatographic and GC-mass spectrometric analyses of body surface extracts of male and female wasps revealed no sex-specific components, but 5-methylheptacosane (5-me-27Hy) and 5,17-dimethylheptacosane (5,17-dime-27Hy) were consistently more abundant in extracts of males. The ratio of 5-me-27Hy and 5,17-dime-27Hy was similar in extracts of males and females, and quantitative differences alone seemed insufficient to impart sex-specific CHC profiles. Therefore, we further hypothesized that the absolute configuration of 5-me-27Hy and 5,17-dime-27Hy contributes to mate recognition or attraction. As the stereoisomers of 5-me-27Hy and 5,17-dime-27Hy cannot currently be separated chromatographically, we could not determine the stereochemistry of the insect-produced components. Instead, we synthesized all stereoisomers and bioassayed synthetic blends in laboratory experiments. Of eight 2-component blends, each blend containing one of the two enantiomers of 5-me-27Hy and one of the four stereoisomers of 5,17-dime-27Hy, the blend of (5S)-methylheptacosane and (5R,17S)-dimethylheptacosane attracted males, whereas the blend of (5R)-methylheptacosane and (5R,17R)-dimethylheptacosane repelled males. Apparent recognition of both pheromone components and pheromone antagonists by males supports the hypothesis that the stereochemistry of 5-me-27Hy and 5,17-dime-27Hy, and possibly other methylated CHCs, may differ between male and female O. kuvanae, and that these differences may serve in mate attraction and recognition.








Similar content being viewed by others
References
Ablard, K., Fairhurst, S., and ersen, G., Schaefer, P., and Gries, G. 2011. Mechanisms, functions, and fitness consequences of pre- and post-copulatory rituals of the parasitoid wasp Ooencyrtus kuvanae. Entomol. Exp. Appl. 140:103–111.
Ardeh, M. J., de Jong, P. W., Loomans, A. J. M., and Van Lenteren, J. C. 2004. Inter- and intraspecific effects of volatile and nonvolatile sex pheromones on males mating behavior, and hybridization in Eretmocerus mundus and E. eremicus (Hymenoptera: Aphelinidae). J. Insect Behav 17:745–759.
Ayasse, M., Paxton, R., and Tengo, J. 2001. Mating behavior and chemical communication in the order Hymenoptera. Annu. Rev. Entomol. 46:31–78.
Borden, J. H., Chong, L., McLean, J. A., Slessor, K. N., and Mori, K. 1976. Gnathotrichus sulcatus: Synergistic response to enantiomers of aggregation pheromone sulcatol. Science 192:894–896.
Brown, M. W. 1984. Literature review of Ooencyrtus kuvanae [Hym.: Encyrtidae], an egg parasite of Lymantria dispar [Lep: Lymantriidae]. Entomophaga 29:249–265.
Burke, S. D., Cobb, J. E., and Takeuchi, K. 1985. Total synthesis of (+)-Phyllanthocin. J. Org. Chem. 50:3420–3421.
Cardé, R. T., Doane, C. C., Baker, T. C., Iwaki, S., and Marumo, S. 1977. Attractancy of optically active pheromone for male gypsy moth. Environ. Entomol. 6:768–772.
Carlson, D., Mramba, F., Sutton, B., Bernier, U., Geden, C., and Mori, K. 2005. Sex pheromone of the tsetse species, Glossina austeni: Isolation and identification of natural hydrocarbons, and bioassay of synthesized compounds. Med. Vet. Entomol. 19:470–479.
Decker, U., Powell, W., and Clark, S. 1993. Sex pheromones in the cereal aphid parasitoids Praon volucre and Aphidius rhopalosiphi. Entomol. Exp. Appl. 69:33–39.
Delury, N., Gries, G., Gries, R., Judd, G., and Brown, J. 1999. Sex pheromone of Ascogaster quadridentata, a parasitoid of Cydia pomonella. J. Chem. Ecol. 25:2229–2245.
Duff, C., Gries, G., Mori, K., Shirai, Y., Seki, M., Takikawa, H., Sheng, T., Slessor, K., Gries, R., Maier, C., and Ferguson, D. 2001. Does pheromone biology of Lambdina athasaria and L. pellucidaria contribute to their reproductive isolation? J. Chem. Ecol. 27:431–442.
Eller, F. J., Bartelt, R. J., Jones, R. L., and Kulman, H. M. 1984. Ethyl (Z)-9-hexadecenoate a sex pheromone of Syndipnus rubiginosus, a sawfly parasitoid. J. Chem. Ecol. 10:291–300.
Fauvergue, X., Hopper, K., and Antolin, M. 1995. Mate finding via a trail sex-pheromone by a parasitoid wasp. Proc. Natl. Acad. Sci. USA 92:900–904.
Ginzel, M., Millar, J., and Hanks, L. 2003. (Z)-9-Pentacosene - contact sex pheromone of the locust borer, Megacyllene robiniae. Chemoecology 13:135–141.
Godfray, H. C. J. 1994. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press, Princeton.
Gries, G., Gries, R., Krannitz, S., Li, J., King, G., Slessor, K., Borden, J., Bowers, W., West, R., and Underhill, E. 1993a. Sex pheromone of the western hemlock looper, Lambdina fiscellaria lugubrosa (Hulst) (Lepidoptera: Geometridae). J. Chem. Ecol. 19:1009–1019.
Gries, G., King, G., Gries, R., Wimalaratne, P., Gray, T., Shepherd, R., Li, J., Slessor, K., and Khaskin, G. 1993b. 3,13-Dimethylheptadecane: Major sex pheromone component of the western false hemlock looper, Nepytia freemani Munroe (Lepidoptera: Geometridae). J. Chem. Ecol. 19:1501–1510.
Gries, G., Clearwater, J., Gries, R., Khaskin, G., King, S., and Schaefer, P. 1999. Synergistic sex pheromone components of white-spotted tussock moth, Orgyia thyellina. J. Chem. Ecol. 25:1091–1104.
Gries, R., Gries, G., Li, J., Maier, C., Lemmon, C., and Slessor, K. 1994. Sex pheromone components of the spring hemlock looper, Lambdina athasaria (Walker) (Lepidoptera: Geometridae). J. Chem. Ecol. 20:2501–2511.
Gries, R., Gries, G., King, G., and Maier, C. 1997. Sex pheromone components of the apple leafminer, Lyonetia prunifoliella. J. Chem. Ecol. 23:1119–1130.
Gries, R., Khaskin, G., Khaskin, E., Foltz, J., Schaefer, P., and Gries, G. 2003. Enantiomers of (Z,Z)-6,9-heneicosadien-11-ol: Sex pheromone components of Orgyia detrita. J. Chem. Ecol. 29:2201–2212.
Gries, R., Khaskin, G., Bennett, R., Miroshnychenko, A., Burden, K., and Gries, G. 2005. (S,S)-2,12-, (S,S)-2,13-, and (S,S)-2,14-Diacetoxyheptadecanes: Sex pheromone components of red cedar cone midge, Mayetiola thujae. J. Chem. Ecol. 31:2933–2946.
Gulias Gomes, C. C., Trigo, J. R., and Eiras, A. E. 2008. Sex pheromone of the american warble fly, Dermatobia hominis: The role of cuticular hydrocarbons. J. Chem. Ecol. 34:636–646.
Hofstetter, R. W. and Raffa, K. F. 1997. Effects of host diet on the orientation, development, and subsequent generations of the gypsy moth (Lepidoptera: Lymantriidae) egg parasitoid Ooencyrtus kuvanae (Hymenoptera: Encyrtidae). Environ. Entomol. 26:1276–1282.
Howard, R. 1992. Comparative analysis of cuticular hydrocarbons from the ectoparasitoids Cephalonomia waterstoni and Laelius utilis (Hymenoptera: Bethylidae) and their respective hosts, Cryptolestes ferrugineus (Coleoptera: Cucujidae) and Trogoderma variabile (Coleoptera: Dermestidae). Ann. Entomol. Soc. Am. 85:317–325.
Howard, R. and Liang, Y. 1993. Cuticular hydrocarbons of winged and wingless morphs of the ectoparasitoid Choetospila elegans Westwood (Hymenoptera: Pteromalidae) and its host, larval lesser grain borer (Rhyzopertha dominica) (Coleoptera: Bostrichidae). Comp. Biochem. Physiol. 106:407–414.
Howard, R. and Blomquist, G. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50:371–393.
Jallon, J. and David, J. 1987. Variations in cuticular hydrocarbons among the 8 species of the Drosophila melanogaster subgroup. Evolution 41:294–302.
Johansson, B. G. and Jones, T. M. 2007. The role of chemical communication in mate choice. Biol. Rev. 82:265–289.
King, G. G. S., Gries, R., Gries, G., and Slessor, K. N. 1995. Optical isomers of 3,13-dimethylheptadecane: Sex pheromone components of the western false hemlock looper, Nepytia freemani (Lepidoptera: Geometridae). J. Chem. Ecol. 21:2027–2045.
Klimetzek, D., Loskant, G., Vité, J. P., and Mori, K. 1976. Disparlure: Differences in pheromone perception between gypsy moth and nun moth. Naturwissenschaften 63:581–582.
Kühbandner, S., Sperling, S., Mori, K., and Ruther, J. 2012. Deciphering the signature of cuticular lipids with contact sex pheromone function in a parasitic wasp. J. Exp. Biol. 215:2471–2478.
Leal, W. 1996. Chemical communication in scarab beetles: Reciprocal behavioral agonist-antagonist activities of chiral pheromones. Proc. Natl. Acad. Sci. USA 93:12112–12115.
Marsden, S. P. and Newton, R. 2007. Electrophile-directed diastereoselective alkylation of prochiral enediolates. J. Am. Chem. Soc. 129:12600–12601.
Marshall, J. A., Grote, J., and Audia, J. E. 1987. Acyclic stereocontrol in catalyzed intramolecular Diels-Alder cyclizations leading to octahydronaphtalenecarboxaldehydes. J. Am. Chem. Soc. 109:1186–1194.
Marukawa, K., Takikawa, H., and Mori, K. 2001. Pheromone Synthesis. Part 207. Synthesis of the enantiomers of some methyl-branched cuticular hydrocarbons of the ant, Diacamma sp. Biosci. Biotech. Biochem. 65:305–314.
Matsuyama, K. and Mori, K. 1994. Synthesis of a stereomeric mixture of 13,25-, 11,21- and 11,23-dimethylheptatriacontane, the contact sex pheromone of the tsetse fly, Glossina tachinoides. Biosci. Biotech. Biochem. 58:539–543.
McClure, M., Whistlecraft, J., and McNeil, J. N. 2007. Courtship behavior in relation to the female sex pheromone in the parasitoid Aphidius ervi (Hymenoptera: Braconidae). J. Chem. Ecol. 33:1946–1959.
Millar, J., Giblin, M., Barton, D., and Underhill, E. 1991. Chiral lepidopteran sex attractants: Blends of optically active C20 and C21 diene epoxides as sex attractants for geometrid and noctuid moths (Lepidoptera). Environ. Entomol. 20:450–457.
Miller, J. R., Mori, K., and Roelofs, W. L. 1977. Gypsy moth field trapping and electroantennogram studies with pheromone enantiomers. J. Insect Physiol. 23:1447–1454.
Mohamed, M. and Coppel, H. 1987. Pheromonal basis of courtship behavior in 2 gypsy moth parasitoids: Brachymeria intermedia (Nees) and Brachymeria lasus (Walker) (Hymenoptera: Chalcididae). J. Chem. Ecol. 13:1099–1113.
Mori, K. and Jiang, W. 1992. Pheromone synthesis. CXXXIV. Synthesis of the four possible stereoisomers of 13,17-dimethylnonatriacontane, a kairomone for the wasp Trichogramma nubilale. Liebigs Ann. Chem. 1992:83–85.
Mullen, S. P., Millar, J. G., Schal, C., and Shaw, K. L. 2008. Identification and characterization of cuticular hydrocarbons from a rapid species radiation of Hawaiian swordtailed crickets (Gryllidae: Trigonidiinae: Laupala). J. Chem. Ecol. 34:198–204.
Nakamura, Y. and Mori, K. 2000. Pheromone synthesis, CCIV. Synthesis of the enantiomers of anti-2,6-dimethylheptane-1,7-diol monotetrahydropyranyl ether and their conversion into the enantiomers of the sex pheromone components of the apple leafminer, Lyonetia prunifoliella. Eur. J. Org. Chem. 2000:2745–2753.
Nichols Jr, W. J., Cosse, A. A., Bartelt, R. J., and King, B. H. 2010. Methyl 6-methylsalicylate: A female-produced pheromone component of the parasitoid wasp Spalangia endius. J. Chem. Ecol. 36:1140–1147.
Oehlschlager, A. C., King, G. G. S., Pierce, H. D., Pierce, A. M., Slessor, K. N., Millar, J. G., and Borden, J. H. 1987. Chirality of macrolide pheromones of grain beetles in the genera Oryzaephilus and Cryptolestes and its implications for species specificity. J. Chem. Ecol. 13:1543–1554.
Oguma, Y., Nemoto, T., and Kuwahara, Y. 1992. A sex pheromone study of a fruit fly Drosophila virilis Sturtevant (Diptera: Drosophilidae): additive effect of cuticular alkadienes to the major sex pheromone. Appl. Entomol. Zool. 27:499–505.
Plimmer, J., Schwalbe, C., Paszek, E., Bierl, B., Webb, R., Marumo, S., and Iwaki, S. 1977. Contrasting effectiveness of (+) and (-) enantiomers of disparlure for trapping native populations of gypsy moth (Lepidoptera: Lymantriidae) in Massachusetts. Environ. Entomol. 6:518–522.
Pompanon, F., Deschepper, B., Mourer, Y., Fouillet, P., and Bouletreau, M. 1997. Evidence for a substrate-borne sex pheromone in the parasitoid wasp Trichogramma brassicae. J. Chem. Ecol. 23:1349–1360.
Ruther, J., Doering, M., and Steiner, S. 2011. Cuticular hydrocarbons as contact sex pheromone in the parasitoid Dibrachys cavus. Entomol. Exp. Appl. 140:59–68.
Schlamp, K., Gries, R., Khaskin, G., Brown, K., Khaskin, E., Judd, G., and Gries, G. 2005. Pheromone components from body scales of female Anarsia lineatella induce contacts by conspecific males. J. Chem. Ecol. 31:2897–2911.
Shu, S. and Jones, R. 1993. Evidence for a multicomponent sex-pheromone in Eriborus terebrans (Gravenhorst) (Hym.: Ichneumonidae), a larval parasitoid of the European corn borer. J. Chem. Ecol. 19:2563–2576.
Silk, P. J., Sweeney, J., Wu, J., Sopow, S., Mayo, P. D., and Magee, D. 2011. Contact sex pheromones identified for two species of longhorned beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the subfamily Spondylidinae. Environ. Entomol. 40:714–726.
Singer, T. 1998. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 38:394–405.
Somjee, U., Ablard, K., Crespi, B., Schaefer, P. W., and Gries, G. 2011. Local mate competition in the solitary parasitoid wasp Ooencyrtus kuvanae. Behav. Ecol. Sociobiol. 65:1071–1077.
Steiner, S., Hermann, N., and Ruther, J. 2006. Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis. J. Chem. Ecol. 32:1687–1702.
Sullivan, B. 2002. Evidence for a sex pheromone in bark beetle parasitoid Roptrocerus xylophagorum. J. Chem. Ecol. 28:1045–1063.
Syvertsen, T., Jackson, L., Blomquist, G., and Vinson, S. 1995. Alkadienes mediating courtship in the parasitoid Cardiochiles nigriceps (Hymenoptera: Braconidae). J. Chem. Ecol. 21:1971–1989.
Szöcs, G., Tóth, M., Francke, W., Schmidt, F., Philipp, P., König, W., Mori, K., Hansson, B., and Löfstedt, C. 1993. Species discrimination in 5 species of winter-flying geometrids (Lepidoptera) based on chirality of semiochemicals and flight season. J. Chem. Ecol. 19:2721–2735.
Tadaaki, I., Kusomoto, G., and Hyama, T. 1996. Preparation of optically active alkanediol bis (arylcarboxylic acid) esters as liquid crystals for liquid crystal compositions and display devices. Patent JP 08-217728, Aug 27, 1996.
Takahashi, S. and Sugai, T. 1982. Mating behavior of the parasitoid wasp Tetrastichus hagenowii (Hymenoptera: Eulophidae). Entomol. Gen. 7:287–293.
Thomas, E. J. and Whitehead, J. W. F. 1989. Cytochalasan synthesis: total synthesis of cytochalasin H. J. Chem. Soc. Perkin Trans 1:507–518.
Tóth, M., Helmchen, G., Leikauf, U., Sziraki, G., and Szöcs, G. 1989. Behavioral activity of optical isomers of 5,9-dimethylheptadecane, the sex-pheromone of Leucoptera-scitella L (Lepidoptera: Lyonetidae). J. Chem. Ecol. 15:1535–1543.
Trabalon, M., Campan, M., Clement, J., Lange, C., and Miquel, M. 1992. Cuticular hydrocarbons of Calliphora vomitoria (Diptera): Relation to age and sex. Gen. Comp. Endocrinol. 85:208–216.
van den Assem, J. 1986. Mating behaviour in parasitic wasps, pp. 137–167, in J. Waage and D. Greathead (eds.), Insect Parasitoids. Academic, London, United Kingdom.
van den Assem, J. 1996. Mating behaviour, pp. 163–221, in M. Jervis and N. Kidd (eds.), Insect natural enemies: Practical approaches to their study and evaluation. Springer, The Netherlands.
van den Dool, H. and Kratz, P. 1963. A generalization of retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 11:463–471.
Widemo, F. and Johansson, B. G. 2006. Male-male pheromone signalling in lekking Drosophilia. P. R. Soc. B. 273:713–717.
Acknowledgements
We thank G. Andersen, M. Andersen, H. Bottomley, U. Somjee, and O. Moeri for technical assistance; E. Kiehlmann for review of the manuscript, S. DeMuth for graphical illustrations, and two anonymous reviewers for constructive comments. Funding was provided by a Natural Sciences and Engineering Research Council of Canada (NSERC) – Discovery Grant and by an NSERC – Industrial Research Chair to G. G., with Contech Enterprises, SC Johnson Canada, and Global Forest Science (GF-18-2007-226; GF-18-2007-227) as sponsors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ablard, K., Gries, R., Khaskin, G. et al. Does the Stereochemistry of Methylated Cuticular Hydrocarbons Contribute to Mate Recognition in the Egg Parasitoid Wasp Ooencyrtus kuvanae?. J Chem Ecol 38, 1306–1317 (2012). https://doi.org/10.1007/s10886-012-0189-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0189-4