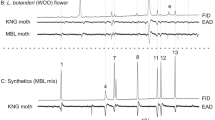
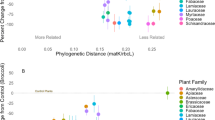
Abstract
Odor is a key trait for pollinator attraction in flowering plants, and many studies have investigated odor evolution in the light of pollinator selection by emphasizing the importance of the plant phylogenetic history. By contrast, little is known on the evolution of odors in fungus–insect interactions. In this study, profiles of three volatile compounds that are emitted by grass-inhabiting Epichloë fungi (Clavicipitaceae, Ascomycota) and that have a confirmed or likely role in the attraction of gamete-transferring Botanophila flies were investigated. We collected headspace samples from stromata of six European Epichloë species (including various host races) that originated from different locations in Switzerland, France, Poland, and UK for conducting gas chromatography analyses. Odor profiles exhibited considerable variation, but profiles of most species overlapped and did not discriminate at the species level. The exception was Epichloë festucae, which had a profile dominated by methyl (Z)-3-methyldodec-2-enoate. Based on an Epichloë phylogeny, there was some hierarchical structuring regarding levels of chokol K, another confirmed Botanophila attractant. However, patterns of odor profiles appeared to be largely dependant on particular Epichloë–host associations. The observed variation may be the result of complex selective pressures imposed by Botanophila gametic vectors, local environment, and mycoparasites.




Similar content being viewed by others
References
Agee, C. S., and Hill, N. S. 1994. Ergovaline variability in Acremonium-infected tall fescue due to environment and plant genotype. Crop Sci. 34:221–226.
Azuma, H., Toyota, M., Asakawa, Y., Yamaoka, R., Garcia-franco, J. G., Dieringer, G., Thien, L. B., and Kawano, S. 1997. Chemical divergence in floral scents of Magnolia and allied genera (Magnoliaceae). Plant Spec. Biol. 12:69–83.
Barkman, T. J. 2001. Character coding of secondary chemical variation for use in phylogenetic analyses. Biochem. Syst. Ecol. 29:1–20.
Bultman, T. L., and Leuchtmann, A. 2003. A test of host specialization by insect vector as a mechanism for reproductive isolation among entomophilous fungal species. Oikos. 103:681–687.
Bultman, T. L., White, J. F., Bowdish, T. I., Welch, A. M., and Johnston, J. 1995. Mutualistic transfer of Epichloë-spermatia by Phorbia flies. Mycologia. 87:182–189.
Bultman, T. L., White, J. F., Bowdish, T. I., and Welch, A. M. 1998. A new kind of mutualism between fungi and insects. Mycol. Res. 102:235–238.
Bultman, T. L., Welch, A. M., Boning, R. A., and Bowdish, T. I. 2000. The cost of mutualism in a fly–fungus interaction. Oecologia. 124:85–90.
Craven, K. D., Hsiau, P. T. W., Leuchtmann, A., Hollin, W., and Schardl, C. L. 2001. Multigene phylogeny of Epichloë species, fungal symbionts of grasses. Ann. Missouri Bot. Gard. 88:14–34.
Dudareva, N., Negre, F., Nagegowda, D. A., and Orlova, I. 2006. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 25:417–440.
Easton, H. S., Latch, G. C. M., Tapper, B. A., and Ball, O. J. P. 2002. Ryegrass host genetic control of concentrations of endophyte-derived alkaloids. Crop Sci. 42:51–57.
Faeth, S. H., Bush, L. P., and Sullivan, T. J. 2002. Peramine alkaloid variation in Neotyphodium-infected Arizona fescue: Effects of endophyte and host genotype and environment. J. Chem. Ecol. 28:1511–1526.
Faeth, S. H., Gardner, D. R., Hayes, C. J., Jani, A., Wittlinger, S. K., and Jones, T. A. 2006. Temporal and spatial variation in alkaloid levels in Achnatherum robustum, a native grass infected with the endophyte Neotyphodium. J. Chem. Ecol. 32:307–324.
Grison-pige, L., Hossaert-mckey, M., Greeff, J. M., and Bessiere, J. M. 2002. Fig volatile compounds—a first comparative study. Phytochemistry. 61:61–71.
Guevara, R., Rayner, A. D. M., and Reynolds, S. E. 2000. Orientation of specialist and generalist fungivorous ciid beetles to host and non-host odors. Physiol. Entomol. 25:288–295.
Hedlund, K., Bengtsson, G., and Rundgren, S. 1995. Fungal odor discrimination in two sympatric species of fungivorous collembolans. Funct. Ecol. 9:869–875.
Hill, N. S., Parrott, W. A., and Pope, D. D. 1991. Ergopeptine alkaloid production by endophytes in a common tall fescue genotype. Crop Sci. 31:1545–1547.
Huber, F. K., Kaiser, R., Sauter, W., and Schiestl, F. P. 2005. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia. 142:564–575.
Knudsen, J. T. 2002. Variation in floral scent composition within and between populations of Geonoma macrostachys (Arecaceae) in the western Amazon. Am. J. Bot. 89:1772–1778.
Knudsen, J. T., Eriksson, R., Gershenzon, J., and Stahl, B. 2006. Diversity and distribution of floral scent. Bot. Rev. 72:1–120.
Koh, S., and Hik, D. S. 2007. Herbivory mediates grass–endophyte relationships. Ecology. 88:2752–2757.
Koshino, H., Yoshihara, T., Togiya, S., Terada, S., Tsukada, S., Okuno, M., Noguchi, A., Sakamura, S., Ichihara, A., Shimanuki, T., Tajimi, A., and Sato, T. 1989. Antifungal compounds from stromata of Epichloë typhina on Phleum pratense, pp. 244–251. Proceedings 31. Symposium on the Chemistry of Natural Products. Sapporo, Japan.
Lembicz, M. 1998. Life history of Puccinellia distans (L.) Parl. (Poaceae) in the colonisation of anthropogenic habitats. Phytocoenosis (N.S.). 10:1–32.
Leuchtmann, A. 2003. Taxonomy and diversity of Epichloë endophytes, pp. 169–194, in J. F. White Jr, C. W. Bacon, N. L. Hywel-Jones, and J. W. Spatafora (eds.). Clavicipitalean FungiMarcel Dekker, New York.
Leuchtmann, A. 2007. Botanophila flies on Epichloë host species in Europe and North America: no evidence for co-evolution. Entomol. Exp. Appl. 123:13–23.
Levin, R. A., Raguso, R. A., and Mcdade, L. A. 2001. Fragrance chemistry and pollinator affinities in Nyctaginaceae. Phytochemistry. 58:429–440.
Levin, R. A., Mcdade, L. A., and Raguso, R. A. 2003. The systematic utility of floral and vegetative fragrance in two genera of Nyctaginaceae. Syst. Biol. 52:334–351.
Olejniczak, P., and Lembicz, M. 2007. Age-specific response of the grass Puccinellia distans to the presence of a fungal endophyte. Oecologia. 152:485–494.
Pellmyr, O., and Thien, L. B. 1986. Insect reproduction and floral fragrances—keys to the evolution of the angiosperms. Taxon. 35:76–85.
Plepys, D., Ibarra, F., Francke, W., and Lofstedt, C. 2002. Odor-mediated nectar foraging in the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae): Behavioural and electrophysiological responses to floral volatiles. Oikos. 99:75–82.
Posada, D., and Crandall, K. A. 1998. MODELTEST: Testing the model of DNA substitution. Bioinformatics. 14:817–818.
R Development Core Team. 2005. R: A Language and Environment for Statistical Computing. Vienna.
Raguso, R. A., and Roy, B. A. 1998. ‘Floral’ scent production by Puccinia rust fungi that mimic flowers. Mol. Ecol. 7:1127–1136.
Raguso, R. A., Levin, R. A., Foose, S. E., Holmberg, M. W., and Mcdade, L. A. 2003. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry. 63:265–284.
Rao, S., and Baumann, D. 2004. The interaction of a Botanophila fly species with an exotic Epichloë fungus in a cultivated grass: fungivore or mutualist? Entomol. Exp. Appl. 112:99–105.
Roylance, J. T., Hill, N. S., and Agee, C. S. 1994. Ergovaline and peramine production in endophyte-infected tall fescue: Independent regulation and effects of plant and endophyte genotype. J. Chem. Ecol. 20:2171–2183.
Salzmann, C. C., Brown, A., and Schiestl, F. P. 2006. Floral scent emission and pollination syndromes: Evolutionary changes from food to sexual deception. Int. J. Plant Sci. 167:1197–1204.
Schardl, C. L., Leuchtmann, A., Chung, K. R., Penny, D., and Siegel, M. R. 1997. Coevolution by common descent of fungal symbionts (Epichloë spp.) and grass hosts. Mol. Biol. Evol. 14:133–143.
Schardl, C. L., Leuchtmann, A., and Mcdonald, B. A. 2007. Relationships of Epichloë typhina isolates from different host grasses, pp. 451–455, in A. J. Popay, and E. R. Thom (eds.). Proceedings of the 6th International Symposium on Fungal Endophytes of GrassesNew Zealand Grassland Association (Inc.), Dunedin, New Zealand.
Schiestl, F. P., Steinebrunner, F., Schulz, C., von Reuss, S., Francke, W., Weymuth, C., and Leuchtmann, A. 2006. Evolution of ‘pollinator’-attracting signals in fungi. Biol. Lett. 2:401–404.
Schomburg, G. 1990. Gas Chromatography: A Practical Course. Wiley-VCH, Weinheim, New York.
Steinebrunner, F., Twele, R., Francke, W., Leuchtmann, A., and Schiestl, F. P. 2008a. Role of odor compounds in the attraction of gamete vectors in endophytic Epichloë fungi. New Phytol. 178:401.
Steinebrunner, F., Schiestl, F. P., and Leuchtmann, A. 2008b. Ecological role of volatiles produced by Epichloë: differences in antifungal toxicity. FEMS Microbiol. Ecol. 64:307–316.
Swofford, D. L. 2003. PAUP: Phylogenetic Analysis Using Parsimony and Other Methods. Sinauer, Sunderland, Massachusetts.
Tanaka, A., Tapper, B. A., Popay, A., Parker, E. J., and Scott, B. 2005. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol. Microbiol. 57:1036–1050.
Thompson, J. N. 1999. Specific hypotheses on the geographic mosaic of coevolution. Am. Nat. 153:S1–S14.
Williams, N. H., and Whitten, W. M. 1999. Molecular phylogeny and floral fragrances of male euglossine bee-pollinated orchids: A study of Stanhopea (Orchidaceae). Plant Spec. Biol. 14:129–136.
Young, C. A., Felitti, S., Shields, K., Spangenberg, G., Johnson, R. D., Bryan, G. T., Saikia, S., and Scott, B. 2006. A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet. Biol. 43:679–693.
Zabalgogeazcoa, I., García ciudad, A., Leuchtmann, A., Vázquez de aldana, B. R., and García criado, B. 2008. Effects of choke disease in the grass Brachypodium phoenicoides. Plant Pathol. published online DOI 10.1111/j.1365–3059.2007.01784.x.
Acknowledgments
The authors thank Jakov Bolotin for help with MS analyses and Sophie Karrenberg for statistical advice. This research was funded by Swiss National Science Foundation grant no. 3100A0-101524.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinebrunner, F., Schiestl, F.P. & Leuchtmann, A. Variation of Insect Attracting Odor in Endophytic Epichloë Fungi: Phylogenetic Constrains Versus Host Influence. J Chem Ecol 34, 772–782 (2008). https://doi.org/10.1007/s10886-008-9476-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9476-5