Abstract
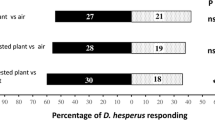
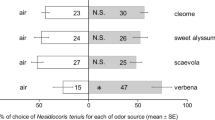
Responses of Neoseiulus cucumeris (a predatory mite) and the predatory insect Orius strigicollis to volatiles associated with two different plant species infested with onion thrips, Thrips tabaci, were examined in a Y-tube olfactometer. Both predators species showed a significant preference for volatiles from infested cucumber leaves without T. tabaci over clean air. However, they were not attracted to volatiles from uninfested cucumber leaves, artificially damaged cucumber leaves, or volatiles from T. tabaci plus their visible products collected from cucumber leaves. These results suggest that both predator species are capable of exploiting herbivore-induced volatiles from T. tabaci-infested cucumber leaves as a foraging cue. Neither predator was attracted to volatiles from uninfested spring onion leaves, infested spring onion leaves without T. tabaci, or volatiles from T. tabaci plus their visible products collected from spring onion leaves. Interestingly, they avoided volatiles from artificially damaged spring onion leaves. A possible explanation for the non-significant olfactory responses of the predator species to spring onion plants with infestation damage of T. tabaci is discussed.


Similar content being viewed by others
References
Aratchige, N. S., Lesna, I., and Sabelis, M. W. 2004. Below-ground plant parts emit herbivore-induced volatiles: olfactory responses of a predatory mite to tulip bulbs infested by rust mites. Exp. Appl. Acarol. 33:21–30.
Auger, J., Dugravot, S., and Thibout, E. 1989. Leek odor analysis by gas chromatography and identification of the most active substance for the leek moth, Acrolepiopsis assectella. J. Chem. Ecol. 15:1847–1854.
Bennison, J., Maulden, K., Dewhirst, S., Pow, E., Slatter, P., and Wadhams, L. 2002. Towards the development of a push–pull strategy for improving biological control of western flower thrips on chrysanthemum, pp. 199–206, in R. Marullo and L. Mound (eds.). Thrips and Tospoviruses. Proceedings of the 7th International Symposium on Thysanoptera, Reggio Calabria, Italy.
Brødsgaard, H. F., and Hansen, L. S. 1992. Effect of Amblyseius cucumeris and Amblyseius barkeri as biological control agents of Thrips tabaci on glasshouse cucumbers. Biocontrol Sci. Technol. 2:215–223.
Childers, C. C. 1997. Feeding and oviposition injuries to plants, pp. 505–537, in T. Lewis (ed.). Thrips as Crop Pests. CAB International, New York.
Choh, Y., Shimoda, T., Ozawa., R., Dicke, M., and Takabayashi, J. 2004. Exposure of lima bean leaves to volatiles from herbivore-infested conspecific plants results in emission of carnivore attractants: active of passive process. J. Chem. Ecol. 30:1305–1317.
De Boer, J. G., Posthumus, M. A., and Dicke, M. 2004. Identification of volatiles that are used in discrimination between plants infested with prey or non–prey herbivores by a predatory mite. J. Chem. Ecol. 30:2215–2230.
De Boer, J. G., Snoeren, T. A., and Dicke, M. 2005. Predatory mites learn to discriminate between plant volatiles induced by prey and nonprey herbivores. Anim. Behav. 69:869–879.
Dicke, M. 1999a. Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl. 91:131–142.
Dicke, M. 1999b. Evolution of induced indirect defense of plants, pp. 63–88, in R. Tollrian, and C. D. Harvell (eds.). The Ecology and Evolution of Inducible Defense, Princeton University Press, Princeton.
Dicke, M., Van Beek, T. A., Posthumus, M. A., Ben Don, N., Van Bokhoven, H., and De Groot, A. E. 1990a. Isolation and identification of volatile kairomone that affects acarine predator–prey interactions: Involvement of host plant in its production. J. Chem. Ecol. 16:381–396.
Dicke, M., Van Der Maas, K. J., Takabayashi, J., and Vet, L. E. M. 1990b. Learning affects response of volatile allelochemicals by predatory mites. Proc. Exp. Appl. Entomol. 1:31–36.
Dicke, M., Takabayashi, J., Posthumus, M. A., Schütte, C., and Olga, E. K. 1998. Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: Variation in production of cues and in responses of predatory mites. Exp. Appl. Acarol. 22:311–333.
Dicke, M., Agrawal, A. A., and Bruin, J. 2003. Plant talk, but are they deaf. Trends Pl. Sci. 8:403–405.
Doederlein, T. A., and Sites, R. W. 1993. Host plant preferences of Frankliniella occidentalis and Thrips tabaci (Thysanoptera: Thripidae) for onions and associated weeds on the Souther High Plains. J. Econ. Entomol. 86:1706–1713.
Doi, M., Zen, S., Okuda, M., Nakamura, H., Kato, K., and Hanada, K. 2003. Leaf necrosis disease of lisianthus (Eustoma ganadiflorum) caused by Iris yellow spots virus. Jpn. J. Phytopathol. 69:181–188. (in Japanese with English summary).
Drukker, B., Scutareanu, P., and Sabelis, M. W. 1995. Do anthocorid predators respond to synomones from Psylla-infested pear trees under field conditions? Entomol. Exp. Appl. 77:193–203.
Drukker, B., Bruin, J., Jacobs, G., Kroon, A., and Sabelis, M. W. 2000a. How predatory mites learn to cope with variability in volatile plant signals in the environment of their herbivorous prey. Exp. Appl. Acarol. 24:881–895.
Drukker, B., Bruin, J., and Sabelis, M. W. 2000b. Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiol. Entomol. 25:260–265.
Dugravot, S., and Thibout, E. 2006. Consequences for a specialist insect and its parasitoid of the response of Allium porrum to conspecific herbivore attack. Physiol. Entomol. 31:73–79.
Dugravot, S., Thibout, E., Abo-Ghalia, A., and Huignard, J. 2004. How a specialist and a non-specialist insect cope with dimethyl disulfide produced by Allium porrum? Entomol. Exp. Appl. 113:173–179.
Dugravot, S., Mondy, N., Mandon, N., and Thibout, E. 2005. Increased sulfur precursors and volatiles production by the leek Allium porrum in response to specialist insect attack. J. Chem. Ecol. 31:1299–1314.
Edelson, J. V., Cartwright, B., and Royer, T. A. 1989. Economics of controlling onion thrips (Thysanoptera: Thripidae) on onions with insecticides in south Texas. J. Econ. Entomol. 82:561–564.
Gillespie, D. R. 1989. Biological control of thrips [Thysanoptera: Thripidae] on greenhouse cucumbers by Amblyseius cucumeris. Entomophaga 34:185–192.
Hinomoto, N., Muraji, M., Noda, T., Shimizu, T., and Kawasaki, K. 2004. Identification of five Orius in Japan by multiplex polymerase chain reaction. Biol. Contol. 31:276–279.
Hori, M., and Harada, H. 1995. Screening plants resistant to green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 30:246–249.
Hoy., C. W., and Glenister., C. S. 1991. Releasing Amblyseius spp. (Acarina: Phytoseiidae) to control Thrips tabaci (Thysanoptera: Thripidae) on cabbage. Entomophaga 36:561–573.
James, D. G., and Price, T. S. 2004. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 30:1613–1628.
Janssen, A., Pallini, A., Venzon, M., and Sabelis, M. W. 1998. Behaviour and indirect interactions in food webs of plant-inhabiting arthropods. Exp. Appl. Acarol. 22:497–521.
Karban, R., and Baldwin, I. T. 1997. Induced Responses to Herbivory. Chicago University Press, Chicago, IL, USA.
Kendall., D. M., and Bjostad, L. B. 1987. Susceptibility of onion growth stages to onion thrips (Thysanoptera: thripidae) damage and mechanical defoliation. Environ. Entomol. 16:859–863.
Kritzman., A., Lampel, M., Raccah, B., and Gera, A. 2001. Distribution and transmission of Iris yellow spot virus. Plant Disease 85:838–842.
Maeda, T., Liu, Y., Ishiwari, H., and Shimoda, T. 2006. Conditioned olfactory responses of a predatory mite, Neoseiulus womersleyi, to volatiles from prey-infested plants. Entomol. Exp. Appl. 121:167–175.
Ozawa, R., Arimura, G., Takabayashi, J., Shimoda, T., and Nishioka, T. 2000. Involvement of jasmonic- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 41:391–398.
Scutareanu, P., Drukker, B., Bruin, J., Posthumus, M. A., and Sabelis, M. W. 1996. Leaf volatiles and polyphenols in pear trees infested by Psylla pyricola. Evidence of simultaneously induced responses. Chemoeology 7:34–38.
Shelton, A. M., Wilsey, W. T., and Schmaedick, M. A. 1998. Management of onion thrips (Thysanoptera: Thripidae) on cabbage by using plant resistance and insecticides. J. Econ. Entomol. 91:329–333.
Shimoda, T., and Dicke, M. 1999. Volatile stimuli related to feeding activity of nonprey caterpillars, Spodoptera exigua, affect olfactory response of the predatory mite, Phytoseiulus persimilis. J. Chem. Ecol. 23:2033–2048.
Shimoda, T., Ozawa, R., Sano, K., Yano, E., and Takabayashi, J. 2005. The involvement of volatile infochemicals from spider mites and from food-plants in prey location of the generalist predatory mite, Neoseiulus californicus. J. Chem. Ecol. 31:2019–2032.
Shiojiri, K., Maeda, T., Arimura, G., Ozawa, R., Shimoda, T., and Takabayashi, J. 2002. Functions of plant infochemicals in tritrophic interactions between plants, herbivores and carnivorous natural enemies. Jpn. J. Appl. Entomol. Zool. 46:117–133 (in Japanese with English summary).
Shipp, J. L., and Wang, K. 2003. Evaluation of Amblyseius cucumeris (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae) for control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomatoes. Biol. Contol. 28:271–281.
Sokal, R. R., and Rohlf, F. J. 1995. Biometry. Freeman, New York. p. 887.
Takabayashi, J., and Dicke, M. 1992. Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol. Exp. Appl. 64:187–193.
Takabayashi, J., and Dicke, M. 1996. Plant–carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1:109–113.
Takabayashi, J., Shimoda, T., Dicke, M., Ashihara, W., and Takafuji, A. 2000. Induced response of tomato plants to injury by green and red strains of Tetranychus urticae. Exp. Appl. Acarol. 24:377–383.
Tsuchiya, M. 2001. Occurrence of blemished fruits caused by Thrips tabaci on Satsuma mandarin cultivated in the greenhouses in Shizuoka Prefecture. Ann. Rept. Kanto Pl. Plot. Soc. 48:153–155 (in Japanese with English summary).
Tsuchiya, M. 2002. Infestation and oviposition of Thrips tabaci (Lindeman) on Satsuma mandarin (Citrus unshiu Marc.) Jpn.. J. Appl. Entomol. Zool. 46:217–224 (in Japanese with English summary).
Turlings, T. C. J., Wäckers, F. L., Vet, L. E. M., Lewis, W. J., and Tumlinson, J. H. 1993. Learning of host-finding cues by Hymenopterous parasitoids, pp. 51–78, in D. R. Papaj, and A. C. Lewis (eds.). Insect Learning. Chapman and Hall, New York.
Venugopal, M. S., and Narayanan, V. 1981. Effects of allition on the green peach ahid (Myzus persicae Sulzer). Int. Pest. Cont. 93:130–131.
Venzon, M., Janssen, A., and Sabelis, M. W. 1999. Attraction of a generalist predator towards herbivore-infested plants. Entomol. Exp. Appl. 93:305–314.
Vet, L. E. M., Lewis, W. J., and Carde, R. T. 1993. Parasitoid foraging and learning, pp. 65–101, in R. T. Carde, and W. J. Bell (eds.). Chemical Ecology of Insects 2. Chapman and Hall, New York.
Wang, C. L., Lee, P. C., and Wu, Y. J. 2001. Field augmentation of Orius strigicollis for the control of thrips in Taiwan. International Seminar on Biological Control of IPEC 141–152.
Williams, M. D. 2001. Biological control of thrips on ornamental crops: interactions between the predatory mite Neoseiulus cucumeris (Acari: Phytoseiidae) and western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on cyclamen. Biocontrol Sci. Technol. 11:41–55.
Yasunaga, T. 1997. The flower bug genus Orius Wolff (Heteroptera: Anthocoridae) from Japan and Taiwan, Part II. Appl. Entomol. Zool. 32:379–386.
Acknowledgments
We thank Junji Takabayashi, Rika Ozawa, and Hiromichi Nitta for their constructive comments during the study. We also express gratitude to Kouichi Yamaguchi for providing us with the predatory mites. This study was supported by a Grant-in-Aid for Scientific Research (No. 19380188) of the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tatemoto, S., Shimoda, T. Olfactory Responses of the Predatory Mites (N eoseiulus cucumeris) and Insects (O rius strigicollis ) to Two Different Plant Species Infested with Onion Thrips (T hrips tabaci) . J Chem Ecol 34, 605–613 (2008). https://doi.org/10.1007/s10886-008-9469-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9469-4