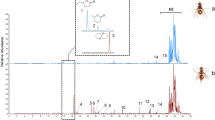
Abstract
The Mediterranean fruit fly, Ceratitis capitata (Wiedemann), displays a lek mating system characterized by a high level of female discrimination among potential mates. The basis of female choice is not understood, but recent studies indicate that male exposure to the aroma of certain plant structures or essential oils may increase mating success. In particular, exposure to the aroma of ginger root oil (GRO) enhances male mating frequency, and several sterile-male release programs against C. capitata have incorporated ‘aromatherapy’ (large-scale exposure of pre-release insects to GRO) to increase the effectiveness of control efforts. We investigated the mechanism underlying female preference for GRO-exposed males. Two sets of experiments were conducted. In the first, we monitored female attraction to (1) freshly killed flies, or (2) paper discs that contained hexane extracts from varying treatments. In these tests, females were sighted more often (1) near GRO-exposed than non-exposed males (even when the males were visually concealed) and (2) near extracts from GRO-exposed than non-exposed males. These findings suggest a ‘perfume effect’, whereby female mate choice is mediated by olfactory differences. In the second set, we compared (1) mate choice between intact females and females from which both antennae had been surgically removed, and (2) mating success between intact males and males from which both antennae had been surgically removed before GRO exposure. Intact females preferred GRO-exposed males, whereas females lacking both antennae rarely mated and showed no preference between GRO-exposed and non-exposed males. In the opposite treatment (intact females but surgically altered males), GRO-exposed males lacking both antennae mated as frequently as GRO-exposed intact males. These data suggest that female choice was dependent on olfactory perception of male odor but that male mating success did not depend on olfactory perception of GRO aroma, suggesting, in turn, that GRO conferred a mating advantage through an external phenomenon (possibly alteration of cuticular scent) rather than through internal processing (pheromone synthesis).


Similar content being viewed by others
References
Amrein, H. 2004. Pheromone perception and behavior in Drosophila. Curr. Opinion Neurobiol 14:435–442.
Andersson, M. 1994. Sexual Selection. Princeton University Press, Princeton, USA.
Arita, L. H., and Kaneshiro, K. Y. 1986. Structure and function of the rectal epithelium and anal glands during mating behavior in the Mediterranean fruit fly male. Proc. Hawaiian Entomol. Soc 26:27–30.
Bartelt, R. J., Schaner, A. M., and Jackson, L. L. 1988. Aggregation pheromones in Drosophila borealis and Drosophila littoralis. J. Chem. Ecol 14:1319–1327.
Bernays, E. A., and Chapman, R. F. 1994. Host-Plant Selection by Phytophagous Insects. Chapman and Hall, New York.
Briceño, D., Eberhard, W. G., and Shelly, T. E. 2007. Male courtship behavior in Ceratitis capitata (Diptera: Tephritidae) that have received aromatherapy with ginger root oil. Fla. Entomol 90:175–179.
Carlson, D. A., Reinert, J. F., Bernier, U. R., Sutton, B. D., and Seawright, J. A. 1997. Analysis of the cuticular hydrocarbons among species of the Anopheles quadrimaculatus complex (Diptera: Culicidae). J. Am. Mosq. Control Assoc Suppl.:103–111.
Carlson, D. A., and Yocom, S. R. 1986. Cuticular hydrocarbons from six species of fruit flies. Arch. Insect Biochem. Physiol 3:397–412.
Connor, W. F., Eisner, T., Vandermeer, R. K., Guerrero, A., and Meinwald, J. 1981. Precopulatory sexual interaction in an arctiid moth (Utetheisa ornatrix): the role of a pheromone derived from dietary alkaloids. Behav. Ecol. Sociobiol 9:227–235.
Coracini, M., Bengtsson, M., Liblikas, I., and Witzgall, P. 2004. Attraction of codling moth males to apple volatiles. Entomol. Exp. Appl 110:1–10.
Costanzo, K., and Monteiro, A. 2007. The use of chemical and visual cues in female choice in the butterfly Bicyclus anynana. Proc. Roy. Soc. Lond. B 274:845–851.
Coyne, J. A., Crittenden, A. P., and Mah, K. 1994. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science 265:1461–1464.
Douglas, M. H., Van Klink, J. W., Smallfield, B. M., Perry, N. B., Anderson, R. E., Johnstone, P., and Weavers, R. T. 2004. Essential oils from New Zealand manuka: triketone and other chemotypes of Leptospermum scoparium. Phytochemistry 65:1255–1264.
Dowd, P. F., and Bartelt, R. J. 1991. Host-derived volatiles as attractants and pheromone synergists for dried fruit beetle, Carpophilus hemipterus. J. Chem. Ecol 17:285–308.
Etges, W. J., and Jackson, L. L. 2001. Epicuticular hydrocarbon variation in Drososphila mojavensis cluster species. J. Chem. Ecol 27:2125–2149.
Féron, M. 1962. L’instinct de reproduction chez la mouche Mediterranéan des fruits Ceratitis capitata Wied. (Diptera: Trypetidae). Comportement sexuel. Comportement de ponte. Rev. Pathol. Veg. Entomol. Agric. Fr 41:1–129.
Flath, R. A., Cunningham, R. T., Mon, T. R., and John, J. O. 1994a. Additional male Mediterranean fruit fly (Ceratitis capitata Wied.) attractants from angelica seed oil (Angelica archangelica L.). J. Chem. Ecol 20:1969–1984.
Flath, R. A., Cunningham, R. T., Mon, T. R., and John, J. O. 1994b. Male lures for Mediterranean fruit fly (Ceratitis capitata Wied.): structural analogues of α-copaene. J. Chem. Ecol 20:2595–2609.
Ginzel, M. D., and Hanks, L. M. 2005. Role of host plant volatiles in mate location for three species of longhorned beetles. J. Chem. Ecol 31:213–217.
Greenfield, M. D., Shelly, T. E., and Downum, K. R. 1987. Variation in host plant quality: implications for territoriality in a desert grasshopper. Ecology 68:828–838.
Hanks, L. M., Millar, J. G., and Paine, T. D. 1996. Mating behavior of the eucalyptus longhorned borer (Coleoptera: Cerambycidae) and the adaptive significance of long “horns”. J. Insect Behav 9:383–393.
Heath, R. R., Landolt, P. J., Tumlinson, J. H., Chambers, D. L., Murphy, R. E., Doolittle, D. E., Dueben, D. B., Sivinski, J., and Calkins, C. O. 1991. Analysis, synthesis, formulation and field testing of the three major components of male Mediterranean fruit fly pheromone. J. Chem. Ecol 17:1925–1940.
Hendrichs, J., and Hendrichs, M. A. 1990. Mediterranean fruit fly (Diptera: Tephritidae) in nature: location and diel pattern of feeding and other activities on fruiting and nonfruiting hosts and nonhosts. Ann. Entomol. Soc. Am 83:632–641.
Hoppe, K. L., Dillwith, J. W., Wright, R. E., and Szumlas, D. E. 1990. Identification of horse flies (Diptera: Tabanidae) by analysis of cuticular hydrocarbons. J. Med. Entomol 27:480–486.
Hovorka, O., Kindl, J., Kalinova, B., Knížek, M., Vrkočová, and Koutek, B. 2005. The role of beetle and host volatiles in host colonization in the European oak bark beetle, Scolytus intricatus (Ratzeburg) (Col., Scolytidae). J. Appl. Entomol 129:221–226.
Hughes, P. R., and Renwick, J. A. A. 1977. Hormonal and host factors stimulating pheromone synthesis in female western pine beetles Dendroctonus brevicomis. Physiol. Entomol 2:289–292.
Jaffe, K., Sanchez, P., Cerda, H., Hernandez, J. A., Jaffe, R., Urdaneta, N., Guerra, G., Martinez, R., and Miras, B. 1993. Chemical ecology of the palm weevil Rhynchophorus palmarum L. (Coleoptera: Curculionidae): attraction to host plants and to a male produced pheromone. J. Chem. Ecol 19:1703–1720.
Jang, E. B., Light, D. M., Binder, R. G., Flath, R. A., and Carvalho, L. A. 1994. Attraction of female Mediterranean fruit flies to the five major components of male-produced pheromone in a laboratory flight tunnel. J. Chem. Ecol 20:9–20.
Jang, E. B., Light, D. M., Flath, R. A., Nagata, J. T., and Mon, T. R. 1989. Electroantennogram responses of the Mediterranean fruit fly, Ceratitis capitata, to identified volatile constituents from calling males. Entomol. Exp. Appl 50:7–19.
Katsoyannos, B. I., Kouloussis, N. A., and Papadopoulos 1997. Response of Ceratitis capitata to citrus chemicals under semi-natural conditions. Entomol. Exp. Appl 82:181–188.
Krasnoff, S. B., and Dussourd, D. E. 1989. Dihydropyrrolizidine attractants for arctiid moths that visit plants containing pyrrolizidine alkaloids. J. Chem. Ecol 15:47–60.
Landolt, P. J., and Phillips, T. W. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol 42:371–391.
Landolt, P. J., Heath, R. R., Millar, J. G., Davis-Hernandez, K. M., Dueben, D. B., and Ward, K. E. 1994. Effects of host plant, Gossypium hirsutum L., on sexual attraction of cabbage looper moths, Trichoplusia ni (Hubner) (Lepidoptera: Noctuidae). J. Chem. Ecol 20:2959–2974.
Levinson, H. Z., Levinson, A. R., and Schäfer, K. 1987. Pheromone biology of the Mediterranean fruit fly (Ceratitis capitata Wied.) with emphasis on the functional anatomy of the pheromone glands and antennae as well as mating behaviour. J. Appl. Entomol 104:448–461.
Lofstedt, C., Vickers, N. J., Roelofs, W. L., and Baker, T. C. 1989. Diet related courtship success in the Oriental fruit moth, Grapholita molesta (Tortricidae). Oikos 55:402–408.
Macleod, A. J., and De Troconis, N. G. 1982. Volatile flavour components of guava. Phytochemistry 21:1339–1342.
Macleod, A. J., Macleod, G., and Subramanian, G. 1988. Volatile aroma constituents of orange. Phytochemistry 27:2185–2188.
Marcillac, F., and Ferveur, J.-F. 2004. A set of female pheromones affects reproduction before, during and after mating in Drosophila. J. Exp. Biol 207:3927–3933.
Moore, A. J. 1988. Female preferences, male social status, and sexual selection in Nauphoeta cinerea. Anim. Behav 36:303–305.
Nakagawa, S., Farias, G. J., Suda, D., and Chambers, D. L. 1973. Mating behavior of the Mediterranean fruit fly following excision of the antennae. J. Econ. Entomol 66:583–584.
Nishida, R., Shelly, T. E., Whittier, T. S., and Kaneshiro, K. Y. 2000. Alpha- copaene, a potential rendezous cue for the Mediterranean fruit fly, Ceratitis capitata?. J. Chem. Ecol 26:87–100.
Papadopoulos, N. T., Katsoyannos, B. I., Kouloussis, N. A., and Hendrichs, J. 2001. Effect of orange peel substances on mating competitiveness of male Ceratitis capitata. Entomol. Exp. Appl 99:253–261.
Papadopoulos, N. T., Shelly, T. E., Niyazi, N., and Jang, E. B. 2006. Olfactory and behavioral mechanisms underlying enhanced mating competitiveness following exposure to ginger root oil and orange oil in males of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J. Insect Behav 19:403–418.
Pivnick, K. A., Lavoie-Dornik, J., and Mcneil, J. N. 1992. The role of the androconia in the mating behaviour of the European skipper, Thymelicus lineola, and evidence for a male sex pheromone. Physiol. Entomol 17:260–268.
Pliske, T. E. 1975. Courtship behavior and use of chemical communication by males of certain species of ithomiinae butterflies (Nymphalidae: Lepidoptera). Ann. Entomol. Soc. Am 68:935–942.
Prokopy, R. J., and Hendrichs, J. 1979. Mating behavior of Ceratitis capitata in field cages on host trees. Ann. Entomol. Soc. Am 72:642–648.
Quiroz, A., Ortega, F., Ramírez, C. C., Wadhams, L. J., and Pinilla, K. 2005. Response of the beetle Hylastinus obscurus Marsham (Coleoptera: Scolytidae) to red clover (Trifolium pretense L.) volatiles in a laboratory olfactometer. Environ. Entomol 34:690–595.
Rantala, M. J., Jokinen, I., Kortet, R., Vainikka, A., and Suhonen 2002. Do pheromones reveal male immunocompetence? Proc. Roy. Soc. Lond. B 269:1681–1685.
Shanbhag, S., Muller, B., and Steinbrecht, A. 1999. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol 28:377–397.
Shelly, T. E. 2001. Exposure to α-copaene and α-copaene-containing oils enhances the mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am 94:497–502.
Shelly, T. E. 2005. Does mating with ginger root oil-exposed males confer fitness benefits to female Mediterranean fruit flies, Ceratitis capitata (Diptera: Tephritidae)? Proc. Hawaiian Entomol. Soc 37:65–71.
Shelly, T. E., and Villalobos, E. 2004. Host plant influence on the mating success of male Mediterranean fruit flies: variable effects within and between individual plants. Anim. Behav 68:417–426.
Shelly, T. E., and Kennelly, S. 2007. Settlement patterns of Mediterranean fruit flies in the tree canopy: an experimental analysis. J. Insect Behav 20:453–472.
Shelly, T. E., Edu, J., Smith, E., Hoffman, K., War, M., Santos, R., Favela, A., Garagliano, R., Ibewiro, B., and Mcinnis, D. O. 2007a. Aromatherapy on a large scale: exposing entire holding rooms to ginger root oil increases the mating competitiveness of sterile males of the Mediterranean fruit fly. Entomol. Exp. Appl 123:193–201.
Shelly, T. E., Cowan, A. N., Edu, J., and Pahio, E. 2007b. Mating success of male Mediterranean fruit flies following exposure to two sources of α-copaene, manuka oil and mango. Fla. Entomol. (in press) .
Shelly, T. E., Dang, C., and Kennelly, S. 2004a. Exposure to orange (Citrus sinensis L.) trees, fruit, and oil enhances mating success of male Mediterranean fruit flies (Ceratitis capitata [Wiedemann]). J. Insect Behav 17:303–315.
Shelly, T. E., Mcinnis, D. O., Pahio, E., and Edu, J. 2004b. Aromatherapy in the Mediterranean fruit fly (Diptera: Tephritidae): sterile males exposed to ginger root oil in pre-release, storage boxes display increased mating competitiveness in field-cage trials. J. Econ. Entomol 97:846–853.
Sivinski, J., Calkins, C. O., and Webb, J. C. 1989. Comparisons of acoustic courtship signals in wild and laboratory reared Mediterranean fruit fly Ceratitis capitata. Fla. Entomol 72:212–214.
Sutton, B. D., and Steck, G. J. 1994. Disrimination of Caribbean and Mediterranean fruit fly larvae (Diptera: Tephritidae) by cuticular hydrocarbon analysis. Fla. Entomol 77:231–237.
Suvanto, L., Liimatainen, J. O., Tregenza, T., and Hoikkala, A. 2000. Courtship signals and mate choice of the flies of inbred Drosophila montana strains. J. Evol. Biol 13:583–592.
Takeoka, G., Flath, R. A., Mon, T. R., Buttery, R. G., Teranishi, R., Guntert, M., Lautamo, R., and Szejtli, J. 1990. Further applications of permethylated β-cyclodextrin capillary gas chromatographic columns. J. High Resol. Chromatogr 13:202–206.
Tanaka, N., Steiner, L. F., Ohinata, K., and Okamoto, R. 1969. Low-cost larval rearing medium for mass-production of oriental and Mediterranean fruit flies. J. Econ. Entomol 62:967–968.
Thornhill, R. 1991. Female preference for the pheromone of males with low fluctuating asymmetry in the Japanese scorpionfly (Panorpa japonica: Mecoptera). Behav. Ecol 3:277–282.
Thornhill, R., and Alcock, J. 1983. The Evolution of Insect Mating Systems. Harvard University Press, Cambridge, USA.
Uebel, E. C., Sonnet, P. E., Menzer, R. E., Miller, R. W., and Lusby, W. R. 1977. Mating-stimulant pheromone and cuticular lipid constituents of the little house fly, Fannia canicularis (L.). J. Chem. Ecol 3:269–278.
Vogt, R. G. 2005. Molecular basis of pheromone detection in insects, pp. 753–804, in L. I. Gilbert, K. Iatro, and S. Gill (eds.). Comprehensive Insect Physiology, Biochemistry, Pharmacology, and Molecular Biology. Vol. 3: EndocrinologyElsevier, London.
Warthen, J. D., and Mcinnis, D. O. 1989. Isolation and identification of male medfly attractive components in Litchi chinensis stems and Ficus spp. stem exudates. J. Chem. Ecol 15:1931–1946.
Whittier, T. S., Nam, F. Y., Shelly, T. E., and Kaneshiro, K. Y. 1994. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera: Tephritidae). J. Insect Behav 7:159–170.
Whittier, T. S., Kaneshiro, K. Y., and Prescott, L. D. 1992. Mating behavior of Mediterranean fruit flies (Diptera: Tephritidae) in a natural environment. Ann. Entomol. Soc. Am 85:214–218.
Acknowledgments
We thank Eric Jang, Jocelyn Millar, David Robacker, and Keng-Hong Tan for technical advice. Sylvia Young of Citrus and Allied Essences Ltd. supplied information regarding the chemical composition of ginger root oil. Eric Jang, Jocelyn Millar, and Boaz Yuval provided comments on an earlier draft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shelly, T.E., Edu, J., Pahio, E. et al. Scented Males and Choosy Females: Does Male Odor Influence Female Mate Choice in the Mediterranean Fruit Fly?. J Chem Ecol 33, 2308–2324 (2007). https://doi.org/10.1007/s10886-007-9394-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9394-y