Abstract
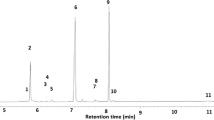
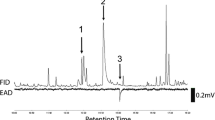
Volatiles from the eggplant flea beetle, Epitrix fuscula Crotch (Coleoptera: Chrysomelidae), feeding on host foliage, were investigated. Six male-specific compounds were detected and were identified through the use of mass spectrometry, nuclear magnetic resonance (NMR) spectrometry, chiral and achiral gas chromatography, high-performance liquid chromatography, electrophysiology (gas chromatography-electroantennography, GC–EAD), and microchemical tests. The two most abundant of the six compounds were (2E,4E,6Z)-2,4,6-nonatrienal (1) and (2E,4E,6E)-2,4,6-nonatrienal (2). The other four compounds, present in minor amounts, were identified as himachalene sesquiterpenes; two of these, 3 and 4, were hydrocarbons and two, 5 and 6, were alcohols. All four sesquiterpenes were previously encountered from male flea beetles of Aphthona spp. and Phyllotreta cruciferae. Synthetic 1 and 2 matched the natural products by GC retention times, mass spectra, and NMR spectra. Sesquiterpenes 3–6 similarly matched synthetic standards and natural samples from the previously studied species in all ways, including chirality. Both natural and synthetic 1 and 2 gave positive GC–EAD responses, as did sesquiterpenes 3, 5, and 6. Field trials were conducted with a mixture of 1 and 2, and the baited traps were significantly more attractive than control traps to both male and female E. fuscula. The E. fuscula pheromone has potential for monitoring or controlling these pests in eggplants.






Similar content being viewed by others
References
Andersen, C. R. 2000. Eggplant. Home Gardening Series. University of Arkansas Cooperative Extension Service. FSA 6010 http://www.uaex.edu/Other_Areas/publications/PDF/FSA-6010.pdf.
Bartelt, R. J., Weisleder, D., and Plattner, R. D. 1990. Synthesis of the nitidulid beetle pheromones: alkyl-branched tetraene hydrocarbons. J. Agric. Food Chem. 38:2192–2196.
Bartelt, R. J., Cossé, A. A., Zilkowski, B. W., Weisleder, D., and Momany, F. A. 2001. Male-specific sesquiterpenes from Phyllotreta and Aphthona flea beetles. J. Chem. Ecol. 27:2397–2423.
Bartelt, R. J., Weisleder, D., and Momany, F. A. 2003. Total synthesis of himachalene sesquiterpenes of Aphthona and Phyllotreta flea beetles. Synthesis 117–123.
Bartelt, R. J., Cossé, A. A., Zilkowski, B. W., Weisleder, D., Grode, S. H., Wiedenmann, R. N., and Post, S. L. 2006. Dimethylfuran–lactone pheromone from males of Galerucella calmariensis and Galerucella pusilla. J. Chem. Ecol. 32:693–712.
Boutagy, J. and Thomas, R. 1974. Olefin synthesis with organic phosphonate carbanions. Chem. Rev. 74:87–99.
Buttery, R. G. 1975. Nona-2,4,6-trienal, an unusual component of blended dry beans. J. Agric. Food Chem. 23:1003–1004.
Capinera, J. L. 2001. Handbook of Vegetable Pests. Academic Press, San Diego, CA.
Cossé, A. A. and Bartelt, R. J. 2000. Male-produced aggregation pheromone of Colopterus truncates: Structure, electrophysiological and behavioral activity. J. Chem. Ecol. 26:1735–1748.
Cossé, A. A., Bartelt, R. J., Zilkowski, B. W., Bean, D. W., and Petroski, R. J. 2005. The aggregation pheromone of Diorhabda elongata, a biological control agent of saltcedar (Tamarix spp.): Identification of two behaviorally active components. J. Chem. Ecol. 31:657–670.
Delahaut, K. 2001. Flea Beetles. University of Wisconsin Garden Facts. University of Wisconsin Extension X1022. <http://www.uwex.edu/ces/wihort/gardenfacts/x1022.pdf>.
Götz-Schmidt, E. and Schreier, P. 1986. Neutral volatiles from blended endive (Cichorium endivia, L.). J. Agric. Food Chem. 34:212–215.
Heath, R. R. and Sonnet, P. E. 1980. Technique for in situ coating of Ag+ onto silica gel in HPLC columns for the separation of geometrical isomers. J. Liq. Chromatogr. 3:1129–1135.
Kreft, A. 1977. Reduction of hindered allylic esters—a cautionary note. Tetrahedron Lett. 23:1959–1960.
Kuepper, G. 2003. Flea Beetle: Organic Control Options. Appropriate Technology Transfer for Rural Areas. March: 1–6 <http://attra.ncat.org/attra-pub/fleabeetle.html>.
Mcleod, P., Diaz, F. J., and Johnson, D. T. 2002. Toxicity, persistence, and efficacy of Spinosad, Chlorfenapyr, and Thiamethoxam on eggplant when applied against the eggplant flea beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 95:331–335.
Mehta, G. and Singh, B. P. 1977. Terpenes and related systems. 16. Fate of representative bicyclic sesquiterpenes in strong acid medium: A general rearrangement of hydroazulene sesquiterpenes to decalin types. J. Org. Chem. 42:632–638.
Miller, A. E. G., Biss, J. W., and Schwartzman, L. H. 1959. Reductions with dialkylaluminum hydrides. J. Org. Chem. 24:627–630.
Mori, K. 2005. Synthesis of (R)-ar-turmerone and its conversion to (R)-ar-himachalene, a pheromone component of the flea beetle: (R)-ar-himachalene is dextrorotatory in hexane, while levorotatory in chloroform. Tetrahedron: Asymmetry 16:685–692.
Muto, S., Bando, M., and Mori, K. 2004. Synthesis and stereochemistry of the four himachalene-type sesquiterpenes isolated from the flea beetle (Aphthona flava) as pheromone candidates. Eur. J. Org. Chem. 2004:1946–1952.
Näf, R. and Velluz, A. 2000. The volatile constituents of extracts of cooked spinach leaves (Spinacia oleracea L.). Flavour Fragr. J. 15:329–334.
Patton, T. W., Dively, G. P., and Miller, A. K. 2003. Evaluation of organic insecticides for the control of flea beetles on eggplant, 2002. Arthropod Management Test 28: E30. <http://www.entsoc.org/Protected/AMT/AMT28/Text/E30.htm>.
Peng, C. and Weiss, M. J. 1992. Evidence of an aggregation pheromone in the flea beetle, Phyllotreta cruciferae (Goeze) (Coleoptera: Chrysomelidae). J. Chem. Ecol. 18:875–884.
Peng, C., Bartelt, R. J., and Weiss, M. J. 1999. Male crucifer flea beetles produce an aggregation pheromone. Physiol. Entomol. 24:98–99.
Petroski, R. J. 2003. Straightforward preparation of (2E,4Z)-2,4-heptadien-1-ol and (2E,4Z)-2,4-heptadienal. Synth. Commun. 33:3233–3241.
Petroski, R. J. and Weisleder D. 2001. Improved Horner-Wadsworth-Emmons preparation of α-methyl- or α-ethyl-α,β-unsaturated esters from aldehydes. Synth. Commun. 31:89–95.
Pivnick, K. A., Lamb, R. J., and Reed, D. 1992. Response of flea beetles, Phyllotreta spp., to mustard oils and nitriles in field trapping experiments. J. Chem. Ecol. 18:863–873.
Schuh, C. and Schieberle, P. 2005. Characterization of (E,E,Z)-2,4,6,-nonatrienal as a character impact aroma compound of oat flakes. J. Agric. Food Chem. 53:8699–8705.
Schuh, C. and Schieberle, P. 2006. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 54:916–924.
Smith, E. H. 1983. Revision of the Genus Phyllotreta Chevrolat of America North of Mexico: Part I. The maculate species (Coleoptera: Chrysomelidae, Alticinae). Fieldiana, Zool. 28:1–168.
Sorensen, K. A. and Baker, J. R. (eds.). 1994. Insect and related pests of vegetables. N. Carolina Cooperative Extension Service. AG295 <http://ipm.ncsu.edu/AG295/html/eggplant_flea_beetle.htm>.
Soroka, J. J., Bartelt, R. J., Zilkowski, B. W., and Cossé, A. A. 2005. Responses of the flea beetle Phyllotreta cruciferae to synthetic aggregation pheromone components and host plant volatiles in field trials. J. Chem. Ecol. 31:1829–1843.
Tóth, M., Csonka, E., Bartelt, R. J., Cossé, A. A., Zilkowski, B. W., Muto, S., and Mori, K. 2005. Pheromonal activity of compounds identified from male Phyllotreta cruciferae: Field tests of racemic mixtures, pure enantiomers, and combinations with allyl isothiocyanate. J. Chem. Ecol. 31:1–16.
Vincent, C. and Stewart, R. K. 1984. Effect of allyl isothiocyanate on field behavior of crucifer-feeding flea beetles (Coleoptera: Chrysomelidae). J. Chem. Ecol. 10:33–39.
Williams, M. A. and Fleming, I. 1980. Spectroscopic Methods in Organic Chemistry, 3rd ed. McGraw-Hill, London.
Wiley. 1995. Wiley. Spectral Library, [CD-ROM], 6th ed. John Wiley & Sons, New York.
Zhang, Z.-Q. and McEvoy, P. B. 1994. Attraction of Longitarsus jacobaeae males to cues associated with conspecific females (Coleoptera: Chrysomelidae). Environ. Entomol. 23:732–737.
Acknowledgments
We are grateful to Anne Patterson of Living Earth Farm for assistance and to Richard Stessman and Nathan Deppe for growing and tending eggplants at the NCAUR garden plots. David Weisleder and Karl Vermillion of NCAUR acquired the NMR spectra. Dr. Alexander S. Konstantinov (Chrysomelidae), Systematic Entomology Laboratory, Agriculture Research Service, US Department of Agriculture, provided insect identification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the Department of Agriculture.
Rights and permissions
About this article
Cite this article
Zilkowski, B.W., Bartelt, R.J., Cossé, A.A. et al. Male-Produced Aggregation Pheromone Compounds from the Eggplant Flea Beetle (Epitrix fuscula): Identification, Synthesis, and Field Biossays. J Chem Ecol 32, 2543–2558 (2006). https://doi.org/10.1007/s10886-006-9163-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9163-3