Abstract
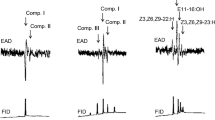
Male Megacyllene caryae (Gahan) (Coleoptera: Cerambycidae) respond to females only after touching them with their antennae, indicating that mate recognition is mediated by a contact sex pheromone. Gas chromatography-mass spectrometry analyses of whole-body solvent extracts of male and female M. caryae revealed substantial differences in hydrocarbon profiles, with nearly half of the compounds in the extracts from females being absent from those of males. Biological activities of fractions of crude extracts of females, and reconstructed blends of the most abundant straight-chain (nC27, nC28, nC29), methyl-branched (2Me-C26, 9Me-C29, 11, 13, 15Me-C29), and unsaturated (Z9:C29, Z13:C29, Z14:C29, Z13:C31, Z14:C31, Z15:C31) compounds in extracts of females were tested in arena bioassays, assessing four steps in the mating behavior sequence of males (orientation, arrestment, body alignment, mounting and attempting to couple the genitalia). Males showed limited response to dead females treated with fractions of the crude extract or blends of synthetic straight-chain and methyl-branched alkanes, but responded strongly to the blend of synthetic monoenes. Further trials determined that the complete sequence of mating behaviors, up to and including coupling the genitalia, was elicited by Z9:C29 alone. Z9:C29 is a homolog of the contact pheromone (Z9:C25) of the congener M. robiniae (Förster). Previous work with M. robiniae suggested that wipe sampling of cuticular hydrocarbons of females by solid phase microextraction yielded a more representative profile of components actually encountered by a male’s antennae, and so provided a more readily interpretable profile of potential semiochemicals present in the wax layer than does solvent extraction. We tested this hypothesis by comparing hydrocarbon profiles of female M. caryae by the two sampling methods. Z9:C29 was the only compound among the dominant hydrocarbons that was present in higher abundance in SPME than in solvent extracts (∼12% vs. ∼8%, respectively), supporting this hypothesis.



Similar content being viewed by others
References
Bland, J. M., Osbrink, W. L. A., Cornelius, M. L., Lax, A. R., and Vigo, C. B. 2001. Solid-phase microextraction for the detection of termite cuticular hydrocarbons. J. Chromatogr. A 932:119–127.
Blomquist, G. J., Tillman-Wall, J. A., Guo, L., Quilici, D. R., Gu, P., and Schal, C. 1993. Hydrocarbon and hydrocarbon derived sex pheromones in insects: Biochemistry and endocrine regulation, pp. 317–351, in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry and Biology. University of Nebraska Press, Lincoln, NE.
Burns, D. H., Miller, J. D., Chan, H. K., and Delaney, M. O. 1997. Scope and utility of a new soluble copper catalyst [CuBr–LiSPh–LiBr–THF]: A comparison with other copper catalysts in their ability to couple one equivalent of a Grignard reagent with an alkyl sulfonate. J. Am. Chem. Soc. 119:2125–2133.
Corey, E. J., Pyne, S. G., and Su, W. 1983. Total synthesis of leukotriene-B5. Tetrahedron Lett. 24:4883–4886.
Coyne, J. A. 1996. Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics 143:1689–1698.
Fukaya, M., Yasuda, T., Wakamura, S., and Honda, H. 1996. Reproductive biology of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). III. Identification of contact sex pheromone on female body surface. J. Chem. Ecol. 22:259–270.
Fukaya, M., Wakamura, S., Yasuda, T., Senda, S., Omata, T., and Fukusaki, E. 1997. Sex pheromonal activity of geometric and optical isomers of synthetic contact pheromone to males of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 32:654–656.
Fukaya, M., Akino, T., Yasuda, T., Wakamura, S., Satoda, S., and Senda, S. 2000. Hydrocarbon components in contact sex pheromone of the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae) and pheromonal activity of synthetic hydrocarbons. Entomol. Science 3:211–218.
Gibbs, A. G. 1998. Water-proofing properties of cuticular lipids. Am. Zool. 38:471–482.
Ginzel, M. D. and Hanks, L. M. 2003. Contact pheromones as mate recognition cues of four species of longhorned beetles (Coleoptera: Cerambycidae). J. Insect Behav. 16:181–187.
Ginzel, M. D. and Hanks, L. M. 2005. Role of host plant volatiles in mate location for three species of longhorned beetles. J. Chem. Ecol. 31:213–217.
Ginzel, M. D., Blomquist, G. J., Millar, J. G., and Hanks, L. M. 2003a. Role of contact pheromones in mate recognition in Xylotrechus colonus. J. Chem. Ecol. 29:533–545.
Ginzel, M. D., Millar, J. G., and Hanks, L. M. 2003b. (Z)-9-Pentacosene-contact sex pheromone of the locust borer, Megacyllene robiniae. Chemoecology 13:135–141.
Kim, G. H., Takabayashi, J., Takahashi, S., and Tabata, K. 1993. Function of contact pheromone in the mating behavior of the cryptomeria bark borer, Semanotus japonicus Lacordaire (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 28:525–535.
Krause, N. and Gerold, A. 1997. Regio- and stereoselective syntheses with organocopper reagents. Angew. Chem., Int. Ed. Engl. 36:187–204.
Lacey, E. S., Ginzel, M. D., Millar, J. G., and Hanks, L. M. 2004. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J. Chem. Ecol. 30:1493–1507.
Liebig, J., Peeters, C., Oldham, N. J., Markstadter, C., and Hölldobler, B. 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl. Acad. Sci. U.S.A. 97:4124–4131.
Linsley, E. G. 1964. The Cerambycidae of North America. Part V. Taxonomy and classification of the subfamily Cerambycinae, tribes Callichromini through Ancylocerini. Univ. Calif. Publ. Entomol. 22:1–197.
Moneti, G., Dani, F. R., Pieraccini, G., and Turillazzi, S. 1997. Solid-phase microextraction of insect epicuticular hydrocarbons for gas chromatographic mass spectrometric analysis. Rapid Commun. Mass. Spectrom. 11:857–862.
Monnin, T., Malosse, C., and Peeters, C. 1998. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant Dinoponera quadriceps. J. Chem. Ecol. 24:473–490.
Nelson, D. R. 1993. Methyl-branched lipids in insects, pp. 271–315, in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry, and Biology. University of Nebraska Press, Lincoln, Nebraska.
Nelson, D. R. and Blomquist, G. J. 1995. Insect waxes, pp. 1–90, in R. J. Hamilton (ed.). Waxes: Chemistry, Molecular Biology, and Function. The Oily Press, Dundee, Scotland.
Nelson, D. R. and Carlson, D. A. 1986. Cuticular hydrocarbons of the tsetse flies Glossina morsitans morsitans, G. austeni, and G. pallidipes. Insect Biochem. 16:403–416.
Page, M., Nelson, L. J., Blomquist, G. J., and Seybold, S. J. 1997. Cuticular hydrocarbons as chemotaxonomic characters of pine engraver beetles (Ips spp.) in the grandicollis subgeneric group. J. Chem. Ecol. 23:1053–1099.
Peeters, C., Monnin, T., and Malosse, C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266:1323–1327.
Roux, E., Sreng, L., Provost, E., Roux, M., and Clement, J. L. 2002. Cuticular hydrocarbon profiles of dominant versus subordinate male Nauphoeta cinerea cockroaches. J. Chem. Ecol. 28:1221–1235.
Sledge, M. F., Moneti, G., Pieraccini, G., and Turillazzi, S. 2000. Use of solid phase microextraction in the investigation of chemical communication in social wasps. J. Chromatogr. A 873:73–77.
Sokal, R. R. and Rohlf, F. J. 1995. Biometry, 3rd edn. Freeman, New York.
Tentschert, J., Bestmann, H. J., and Heinze, J. 2002. Cuticular compounds of workers and queens in two Leptothorax ant species—a comparison of results obtained by solvent extraction, solid sampling, and SPME. Chemoecology 12:15–21.
Turillazzi, S., Sledge, M. F., and Moneti, G. 1998. Use of a simple method for sampling cuticular hydrocarbons from live social wasps. Ethol. Ecol. Evol. 10:293–297.
Wang, Q. 1998. Evidence for a contact female sex pheromone in Anoplophora chinensis (Förster) (Coleoptera: Cerambycidae: Lamiinae). Coleopt. Bull. 52:363–368.
Acknowledgments
We thank the University of Illinois at Urbana–Champaign (UIUC) Committee on Natural Areas for use of Brownfield Woods for our research, and E. S. Lacey, J. Mohler, K. Rinkenberger, and J. Westberg for assistance in rearing and collecting beetles and maintaining colonies. This research was in partial fulfillment of a PhD degree for M.D.G. from UIUC. We appreciate funding support from the Alphawood Foundation of Chicago, the U.S. Forest Service (agreement #01-JV-11231300-088), and grants to MDG from the Herbert Holdsworth Ross Memorial Fund of the Illinois Natural History Survey and the UIUC Graduate College.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ginzel, M.D., Moreira, J.A., Ray, A.M. et al. (Z)-9-Nonacosene—Major Component of the Contact Sex Pheromone of the Beetle Megacyllene caryae . J Chem Ecol 32, 435–451 (2006). https://doi.org/10.1007/s10886-005-9010-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-9010-y