Abstract
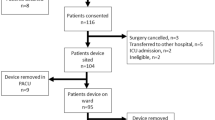
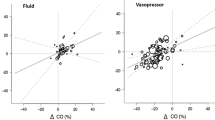
Stable intraoperative haemodynamics are associated with improved outcome and even short periods of instability are associated with an increased risk of complications. During anaesthesia intermittent non-invasive blood pressure and heart rate remains the cornerstone of haemodynamic monitoring. Continuous monitoring of systemic blood pressure or even -flow requires invasive or advanced modalities creating a barrier for obtaining important real-time haemodynamic insight. The Peripheral Perfusion Index (PPI) is obtained continuously and non-invasively by standard photoplethysmography. We hypothesized that changes in indices of systemic blood flow during general anaesthesia would be reflected in the PPI. PPI, stroke volume (SV), cardiac output (CO) and mean arterial pressure (MAP) were evaluated in 20 patients. During general anaesthesia but before start of surgery relative changes of SV, CO and MAP were compared to the relative changes of PPI induced by head-up (HUT) and head-down tilt (HDT). Furthermore, the effect of phenylephrine (PE) during HUT on these parameters was investigated. ∆PPI correlated significantly (p < 0.001) with ∆SV (r = 0.9), ∆CO (r = 0.9), and ∆MAP (r = 0.9). HUT following induction of anaesthesia resulted in a decrease in PPI of 41% (25–52) [median (IQR)], SV 27% (23–31), CO 27% (25–35), and MAP 28% (22–35). HDT led to an increase in PPI of 203% (120–375), SV of 29% (21–41), CO 22% (16–34), and MAP 47% (42–60). After stabilizing a second HUT decreased PPI 59% (49–76), SV 33% (28–37), CO 31% (28–36), and MAP 34% (26–38). Restoration of preload with PE increased PPI by 607% (218–1078), SV by 96% (82–116), CO by 65% (56–99), and MAP by 114% (83–147). During general anaesthesia changes in PPI tracked changes in systemic haemodynamics.


Similar content being viewed by others
References
Sessler DI, Meyhoff CS, Zimmerman NM, Mao G, Leslie K, Vásquez SM, et al. Period-dependent associations between hypotension during and for four days after noncardiac surgery and a composite of myocardial infarction and death. Anesthesiology. 2018;128:317–27.
Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery. Anesthesiology. 2017;126:47–65.
Vincent JL, Pelosi P, Pearse R, Payen D, Perel A, Hoeft A, et al. Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit Care. 2015;19:1–12.
Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system. Anesthesiology. 2018;128:55–66.
Lima AP, Beelen P, Bakker J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med. 2002;30:1210–3.
Reisner A, Shaltis PA, McCombie D, Asada HH. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology. 2008;108:950–8.
Nuttall G, Burckhardt J, Hadley A, Kane S, Kor D, Marienau MS, et al. Surgical and patient risk factors for severe arterial line complications in adults. Anesthesiology. 2016;124:590–7.
Jeleazcov C, Krajinovic L, Münster T, Birkholz T, Fried R, Schüttler J, et al. Precision and accuracy of a new device (CNAPTM) for continuous non-invasive arterial pressure monitoring: assessment during general anaesthesia. Br J Anaesth. 2010;105:264–72.
McCarthy T, Telec N, Dennis A, Griffiths J, Buettner A. Ability of non-invasive intermittent blood pressure monitoring and a continuous non-invasive arterial pressure monitor (CNAP TM) to provide new readings in each 1-min interval during elective caesarean section under spinal anaesthesia. Anaesthesia. 2012;67:274–9.
Schmidt C, Theilmeier G, Van Aken H, Korsmeier P, Wirtz SP, Berendes E, et al. Comparison of electrical velocimetry and transoesophageal Doppler echocardiography for measuring stroke volume and cardiac output. Br J Anaesth. 2005;95:603–10.
Martin E, Anyikam A, Ballas J, Buono K, Mantell K, Huynh-Covey T, et al. A validation study of electrical cardiometry in pregnant patients using transthoracic echocardiography as the reference standard. J Clin Monit Comput. 2016;30:679–86.
van Genderen ME, Paauwe J, de Jonge J, van der Valk RJP, Lima A, Bakker J, et al. Clinical assessment of peripheral perfusion to predict postoperative complications after major abdominal surgery early: a prospective observational study in adults. Crit Care. 2014;18:R114.
Oskay A, Eray O, Dinç SE, Aydın AG, Eken C. Prognosis of critically ill patients in the ED and value of perfusion index measurement: a cross-sectional study. Am J Emerg Med. 2015;33:1042–4.
He H, Liu D, Long Y, Wang X. The peripheral perfusion index and transcutaneous oxygen challenge test are predictive of mortality in septic patients after resuscitation. Crit Care. 2013;17:R116.
He H, Long Y, Liu D, Wang X, Zhou X. Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care. 2015;19:330.
Klijn E, Groeneveld ABJ, van Genderen ME, Betjes M, Bakker J, van Bommel J. Peripheral perfusion index predicts hypotension during fluid withdrawal by continuous veno-venous hemofiltration in critically ill patients. Blood Purif. 2015;40:92–8.
Toyama S, Kakumoto M, Morioka M, Matsuoka K, Omatsu H, Tagaito Y, et al. Perfusion index derived from a pulse oximeter can predict the incidence of hypotension during spinal anaesthesia for caesarean delivery. Br J Anaesth. 2013;111:235–41.
Duggappa DR, Lokesh M, Dixit A, Paul R, Raghavendra Rao RS, Prabha P. Perfusion index as a predictor of hypotension following spinal anaesthesia in lower segment caesarean section. Indian J Anaesth. 2017;61:649–54.
van Genderen ME, Bartels SA, Lima A, Bezemer R, Ince C, Bakker J, et al. Peripheral perfusion index as an early predictor for central hypovolemia in awake healthy volunteers. Anesth Analg. 2013;116:351–6.
Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol. 2009;587:4987–99.
Beurton A, Teboul J-L, Gavelli F, Gonzalez FA, Girotto V, Galarza L, et al. The effects of passive leg raising may be detected by the plethysmographic oxygen saturation signal in critically ill patients. Crit Care. 2019;23:19.
Corsini I, Cecchi A, Coviello C, Dani C. Perfusion index and left ventricular output correlation in healthy term infants. Eur J Pediatr. 2017;176:1013–8.
Janaillac M, Beausoleil TP, Barrington KJ, Raboisson M-J, Karam O, Dehaes M, et al. Correlations between near-infrared spectroscopy, perfusion index, and cardiac outputs in extremely preterm infants in the first 72 h of life. Eur J Pediatr. 2018;177:541–50.
Ginosar Y, Weiniger CF, Meroz Y, Kurz V, Bdolah-Abram T, Babchenko A, et al. Pulse oximeter perfusion index as an early indicator of sympathectomy after epidural anesthesia. Acta Anaesthesiol Scand. 2009;53:1018–26.
Kus A, Gurkan Y, Gormus SK, Solak M, Toker K. Usefulness of perfusion index to detect the effect of brachial plexus block. J Clin Monit Comput. 2013;27:325–8.
Abdelnasser A, Abdelhamid B, Elsonbaty A, Hasanin A, Rady A. Predicting successful supraclavicular brachial plexus block using pulse oximeter perfusion index. Br J Anaesth. 2017;119:276–80.
Şahin ÖF, Tarıkçı Kılıç E, Aksoy Y, Kaydu A, Gökçek E. The importance of perfusion index monitoring in evaluating the efficacy of stellate ganglion blockage treatment in Raynaud’s disease. Libyan J Med. 2018;13:1422666.
Klodell CT, Lobato EB, Willert JL, Gravenstein N. Oximetry-derived perfusion index for intraoperative identification of successful thoracic sympathectomy. Ann Thorac Surg. 2005;80:467–70.
Taneyama C, Goto H, Kohno N, Benson KT, Sasao J, Arakawa K. Effects of fentanyl, diazepam, and the combination of both on arterial baroreflex and sympathetic nerve activity in intact and baro-denervated dogs. Anesth Analg. 1993;77:44–8.
Sellgren J, Pontén J, Wallin BG. Characteristics of muscle nerve sympathetic activity during general anaesthesia in humans. Acta Anaesthesiol Scand. 1992;36:336–45.
Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725–33.
Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology. 2005;103:20–4.
Robinson BJ, Ebert TJ, O’Brien TJ, Colinco MD, Muzi M. Mechanisms whereby propofol mediates peripheral vasodilation in humans. Sympathoinhibition or direct vascular relaxation? Anesthesiology. 1997;86:64–72.
Neukirchen M, Kienbaum P. Sympathetic nervous system. Anesthesiology. 2008;109:1113–31.
Yoshiya I, Shimada Y, Tanaka K. Spectrophotometric monitoring of arterial oxygen saturation in the fingertip. Med Biol Eng Comput. 1980;18:27–32.
Alexander CM, Teller LE, Gross JB. Principles of pulse oximetry: theoretical and practical considerations. Anesth Analg. 1989;69:368–76.
Tusman G, Bohm SH, Suarez-Sipmann F. Advanced uses of pulse oximetry for monitoring mechanically ventilated patients. Anesth Analg. 2017;124:62–71.
Kemps HMC, Thijssen EJM, Schep G, Sleutjes BTHM, De Vries WR, Hoogeveen AR, et al. Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J Appl Physiol. 2008;105:1822–9.
Geerts B, De Wilde R, Aarts L, Jansen J. Pulse contour analysis to assess hemodynamic response to passive leg raising. J Cardiothorac Vasc Anesth. 2011;25:48–52.
Pittman J, Bar-Yosef S, SumPing J, Sherwood M, Mark J. Continuous cardiac output monitoring with pulse contour analysis: a comparison with lithium indicator dilution cardiac output measurement. Crit Care Med. 2005;33:2015–21.
Cannesson M, Jian Z, Chen G, Vu TQ, Hatib F. Effects of phenylephrine on cardiac output and venous return depend on the position of the heart on the Frank-Starling relationship. J Appl Physiol. 2012;113:281–9.
Wodack KH, Graessler MF, Nishimoto SA, Behem CR, Pinnschmidt HO, et al. Assessment of central hemodynamic effects of phenylephrine: an animal experiment. J Clin Monit Comput. 2019;33:377–84.
Kalmar AF, Allaert S, Pletinckx P, Maes J-W, Heerman J, Vos JJ, et al. Phenylephrine increases cardiac output by raising cardiac preload in patients with anesthesia induced hypotension. J Clin Monit Comput. 2018;32:969–76.
Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–48.
Foss NB, Kehlet H. Perioperative haemodynamics and vasoconstriction: time for reconsideration? Br J Anaesth. 2019;123:100–3.
Rebet O, Andremont O, Gérard J-L, Fellahi J-L, Hanouz J-L, Fischer M-O. Preload dependency determines the effects of phenylephrine on cardiac output in anaesthetised patients. Eur J Anaesthesiol. 2016;33:638–44.
Coutrot M, Joachim J, Dépret F, Millasseau S, Nougué H, Matéo J, et al. Noninvasive continuous detection of arterial hypotension during induction of anaesthesia using a photoplethysmographic signal: proof of concept. Br J Anaesth. 2019;122:605–12.
Futier E, Lefrant J-Y, Guinot P-G, Godet T, Lorne E, Cuvillon P, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–57.
Acknowledgements
We wish to thank Masimo, Irvine, CA, USA, for lending the department Radical 7 monitors.
Funding
No external funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the regional ethics committee, De Videnskabsetiske Komiteer for Region Hovedstaden, (Reference Number H-16030081) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was registered at ClinicalTrials.gov (NCT02989441). Processing of personal data was approved by the Danish Data Protection Agency (AHH-2016-095).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Højlund, J., Agerskov, M., Clemmesen, C.G. et al. The Peripheral Perfusion Index tracks systemic haemodynamics during general anaesthesia. J Clin Monit Comput 34, 1177–1184 (2020). https://doi.org/10.1007/s10877-019-00420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00420-x