Abstract
Neuromuscular blockade is usually monitored using train-of-four (TOF) stimulation pattern. A TOF ratio of higher than 90 % is recommended to reduce the risk of adverse effects after anaesthesia. TOF ratio 90 % is used in clinical practice with all different neuromuscular monitors. Kinemyography (KMG) is one commercialized method to obtain numerical TOF values. We compared the KMG data obtained with Datex M-NMT MechanoSensor™ module, to the EMG data collected with Datex ElectroSensor™, during clinical anaesthesia. Ipsilateral comparisons of the sensors were performed in 20 female patients during clinical procedures in propofol–remifentanil anaesthesia. After initial bolus dose of rocuronium (0.6 mg/kg), the spontaneous recovery of TOF ratio and T1 % were monitored. KMG gave higher TOF values than EMG. The difference was significant at KMG TOF values of 40 % or higher. After anaesthetic induction, but before administration of rocuronium, both TOF sensor values drifted from the TOF value of 1.0, showing either significant spontaneous fade (T1 > T4) or tendency of reverse fade (T1 < T4). KMG overestimates the recovery from neuromuscular blockade when compared with EMG. KMG and EMG cannot be used interchangeably, and TOF ratio 90 % cannot be considered as adequate level of recovery with all monitoring devices.
Similar content being viewed by others
1 Introduction
Neuromuscular blockade (NMB) is an integral part of general anaesthesia, routinely monitored as a muscle response to electrical stimulation of a motor nerve. The train-of-four (TOF) ratio, i.e., a measurement of ratio/fade between first and last of four consecutive electrical stimuli is the most popular stimulation pattern used in modern practice. Measurement of fading in each individual series of four stimuli, rather than comparing a single response to a preset value, makes every measurement act as its own reference.
To ensure satisfactory surgical conditions, deep NMB with PTC ≤ 1, TOF count < 1 is often suggested [1]. However, at the end of surgery, to avoid serious adverse effects of incomplete NMB recovery, a TOF ratio > 90 % is widely recommended [2, 3]. Failure to reach a TOF ratio of 90 % before removal of the endotracheal intubation tube is shown to be associated with reduced hypoxic ventilatory responses [4–6], impaired pharyngeal function [7, 2], and lowered upper esophageal sphincter (UES) pressure [7], increasing risk of regurgitation and aspiration of stomach content. Thus, adequate objective intraoperative monitoring of NMB is mandatory to avoid potentially fatal postoperative complications.
There are numerous methods to quantify the TOF ratio: Electromyography (EMG) measures the compound action potential, while mechanomyography (MMG) measures isometric contraction of muscle, acceleromyography (AMG) measures acceleration, and kinemyography (KMG) measures the bending of a special piezoelectric strip.
MMG is the only method directly validated with aforementioned clinical end points [2]. EMG and MMG are shown to compare adequately with each other [8]. Also good correlation between KMG and MMG has been suggested [9, 10]. The correlation between KMG and EMG has been recently studied in two study reports [11, 12].
In this study, our aim was to compare KMG and EMG in patients during clinical propofol-remifentanil-rocuronium anaesthesia. The measures were done during the spontaneous recovery period of rocuronium induced paralysis.
2 Patients and methods
Twenty-seven females, aged 18–65 years, scheduled for operation in supine or lithotomy position, were enrolled in this study. Written informed consent was obtained from all patients. Exclusion criteria were as follows: BMI > 35, ASA classification 3 or higher, neurological/neuromuscular disease or medication, and recovery rate of neuromuscular function over 5 % per minute. The research protocol was approved by the Ethics Committee of our hospital.
The recording system was attached before anaesthetic induction. The patient was lying in a supine position, and her hand was fixed to a metallic mold allowing only the thumb to move freely (Fig. 1). The recording electrodes of the EMG (Datex ElectroSensor™) were attached according to the instructions of the manufacturer [13] (the recording electrode is placed on top of m. adductor pollicis in thenar eminence and the other recording electrode on top of the distal interphalangeal joint of the thumb. The neutral electrode is placed at centerline over the flexor retinaculum at the palmar side of wrist. The KMG sensor (Datex M-NMT MechanoSensor™) was attached to the same hand between thumb and index finger.
Patients were anaesthetized with target controlled infusions (TCI; Orchestra Base Primea, Fresenius Vial, Le Grand Chemin, Brezins, France) of propofol and remifentanil. The pharmacokinetic model of Schnider was used for administration of propofol and that of Minto for administration of remifentanil [14, 15]. Anaesthetics were adjusted according to the clinical needs, and the patients were kept normocapnic (EtCO2 4.0–5.7 kPa) by adjusting the controlled ventilation. Temperature of the monitoring site was maintained within GCRP recommendations (central ≥ 35 °C, surface at the monitoring site ≥ 32 °C) [1].
After anaesthetic induction and the loss of consciousness, but before administration of rocuronium, the NMT module automatically scanned the sufficient current level for supramaximal nerve stimulation. The stable twitch level of the sensor was ensured by waiting for 5 min, and giving the patient one 50-Hz tetanic stimulus of 5 s [1]. The stable level was confirmed afterwards from the log collected from the anaesthesia machine. Though the T1 was stable after aforementioned initial procedures, most of the patients had TOF ratio unequal to 100 % before any NMBA was applied indicating spontaneous fade (T1 > T4) or reverse fade (T1 < T4).
Thereafter, rocuronium (0.6 mg/kg) was given as a single bolus, the patient’s trachea was intubated, and the surgery was allowed to start. After the initial dose of rocuronium, no further doses of neuromuscular blocking agents (NMBA) were given. As the patient spontaneously emerged from the neuromuscular block (NMB), the recovery of muscle strength was monitored with MechanoSensor™; i.e., with the KMG method by 20 s stimulation intervals. Because of the ipsilateral setting, simultaneous measurements with both monitors were not performed. When the repetitive KMG measurements reached TOF level of 10 %, KMG monitoring was interrupted and replaced with the ElectroSensor™ (EMG) measurement. During the EMG monitoring, three consecutive TOF measures were performed at steady 20 s intervals with the aid of EMG. To minimize the time-related bias, the first EMG response value was used in the analyses, while the two subsequent responses served as references. Thereafter, EMG monitoring was again replaced with KMG. Neuromuscular blockade monitoring was continued with KMG, until the KMG TOF ratio reached 20 % level, and EMG monitoring was re-started to collect the respective EMG TOF values. At each ten percent KMG TOF value (i.e., 30, 40, 50 etc.), three EMG TOF responses were recorded. The study was continued to the KMG TOF values up to 90 %. Thereafter, the NMB was adjusted solely according the clinical needs of the operation.
Sample size was based on our preliminary, unpublished data of nine patients indicating a 10 % difference of mean (75.7 vs. 85.8 % between EMG and KMG, respectively), with standard deviation of 13.06 %, thus yielding the need of 27 subjects to show a significant difference between the methods (alpha 0.05, beta 0.2).
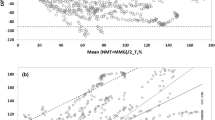
A Bland–Altman plot was used to compare the difference between EMG and KMG against their means [16]. Bland–Altman analysis was made for TOF ratios (Fig. 2) and T1 %. Sign test and t test were used as post hoc analyses of TOF ratios of the subgroups and the spontaneous fade respectively (Microsoft Excel 2007).
3 Results
In this study, KMG overestimated the recovery of TOF%, compared to the data obtained with EMG. The overestimation of KMG TOF% was significant in subgroups during recovery at KMG TOF 40 % or higher (Table 1). Average 95 % limits of agreement in Bland–Altman analysis were 22.53 and −13.92 % for TOF ratios (Fig. 2) and 16.63 and −35.40 % for T1 % comparison. Correlation of repeatability was 3.35 for the difference of the monitors.
At the beginning of the test, before any NMBA was given, both sensors failed to show 100 % TOF ratios in most of the cases, thus showing spontaneous fade (T1 > T4) or reverse fade (T1 < T4). EMG suffered less of this phenomenon, and though it probably is clinically insignificant, the difference was statistically significant (p < 0.0001) (Fig. 3).
Twenty-seven patients were recruited. However, results of seven patients were excluded, resulting in the actual study size of 20 successful recordings (Fig. 4). Reasons for exclusions were failure of the fixation of the hand (tape of the MechanoSensor™) (two patients), unexpectedly fast spontaneous recovery (11 and 6 %/min) of the neuromuscular function, inhibiting the collection of solid and reliable measurements (two patients), failure to achieve supramaximal nerve stimulation (one patient), and interference of simultaneous electrocauterization, which distorted the EMG responses (two patients).
4 Discussion
In this study, kinemyographic measurement of TOF ratio overestimated the recovery compared with EMG. Good correlation between EMG and KMG would have been expected, because earlier studies have shown that both KMG and EMG compares well with MMG [9].
In another study comparing KMG and MMG, KMG was able to reliably predict time to tracheal intubation and full recovery of neuromuscular block [10]. It is noteworthy that if KMG compares well with MMG, it should also be reliable considering clinical endpoints discussed previously.
Two studies comparing EMG and KMG have been previously performed. One study was made with paediatric patients [12] and excellent agreement between KMG and EMG was found. However that study probably cannot be directly extrapolated to an adult population. Another study was performed with an adult population [11] and the results were quite similar to that of ours: KMG was found to overestimate TOF ratios measured with EMG, with variation from 65 to 100 %, according to the authors these two cannot be used interchangeably. Unfortunately, In both of these earlier studies the GCRP [1] protocol was not followed in detail (e.g., avoidance of inhalational anaesthetics).
There is no gold standard in neuromuscular monitoring, but because studies with direct clinical endpoints have been performed with MMG (mechanomyography), it can be considered as reference method from the clinical point of view.
A neuromuscular monitoring device should tell if the neuromuscular block is deep enough for the desired surgical operation, and whether it is safe to extubate the patient’s trachea at the end of the anaesthesia. Therefore, the accuracy and repeatability are crucial.
All neuromuscular monitors rely on electrical stimulation of nerve and measurement of subsequent muscle response. The device used in this study, i.e., Datex MechanoSensor™ (GE Healthcare, Helsinki, Finland) implements bending measurement between the thumb and the index finger. The bending motion of the sensor is thought to be proportional to the force created by the adductor pollicis muscle after supramaximal stimulation of the ulnar nerve. The sensor contains a strip of piezoelectric polymer, which reacts to the change in shape due to bending [13]. Datex MechanoSensor™ measures KMG, and it has been validated in two studies against Mechanomyography (MMG) [9, 10].
Activation of nicotinic receptor in neuromuscular junction causes depolarization and action potential in muscle cell membrane, eventually leading to muscle contraction. It is noteworthy that different monitors quantifying clinical recovery actually measure different steps in the excitation contraction chain, or different modality of force. Mechanomyography directly quantifies (isometric/static) contraction force, whereas acceleromyography, and kinemyography do the same indirectly by assessing acceleration or the extend of bending, which is assumed to be proportional to the (dynamic) force of the muscle. Electromyography relies on measurement of compound action potential of muscle cells, reflecting the function of the neuromuscular junction earlier in the excitation contraction chain.
Both KMG and AMG have shown to correlate poorly with EMG during recovery from neuromuscular paralysis [11, 12, 17]. The fade induced with NMBA is produced in the neuromuscular junction, and the neuromuscular junction most probably do not have direct feedback if the myofibrils are contracting isometrically or dynamically after the action potential in sarcolemma. Thus there is no reasonable physiological explanation in the properties of muscle itself which could explain the difference of fade between dynamic versus isometric contraction. We think that nonlinearities (e.g., nonlinear discordance between the boomerang shaped KMG sensor and the force vector of the muscle) in the behavior of piezoelectric methods (dynamic methods, KMG and AMG) could rather be the cause of aforementioned discrepancy.
Furthermore, as acknowledged, clinical tests which are still used in many institutions are not an adequate option for neuromuscular monitoring in daily practice. In a prospective study of 640 patients, eight clinical tests or a sum of them were unable to predict residual paralysis [18]. Clinical tests, e.g., head lift, also require co-operation, making them useless in anaesthetized patients. When subjective tests (visual or tactile feedback from electrical nerve stimulation) are used, clinicians often are unable to detect fade when (quantifically measured) TOF ratios are between 0.4 and 1.0 [19, 20].
Although all quantitative methods, like electromyography (EMG), mechanomyography (MMG), kinemyography (KMG), acceleromyography (AMG) etc., are superior to clinical tests, the measures are not directly comparable with each other. Therefore it is rational to question, whether the acknowledged recovery level for safe extubation of endotracheal tube (i.e., TOF 0.9), reflects the same physiological state with all the different methods.
According to this study, if the anesthesiologist using KMG follows good practice, performing extubation at KMG TOF level of 0.9, the simultaneous EMG TOF may vary between 0.76 and 0.95. Referring to EMG data, 70 % of our research patients were not successfully recovered (i.e., EMG TOF < 0.9 at the time of extubation at KMG TOF 0.9).
We made a direct comparison between EMG and KMG data and found significant differences in TOF ratios. Whether this actually has clinical significance or not remains unclear, because we did not have simultaneous mechanomyographic control, and indirect assessment of safe extubation levels would be more or less speculative. One must be careful in making indirect comparisons between different monitoring methods, because there are differences between research protocols and question settlements.
After the stable response was achieved, Datex MechanoSensor™ produced notable spontaneous fade (T1 > T4), or even reverse fade (T1 < T4), before rocuronium was given. The Difference between sensors was significant, while EMG was less prone to this phenomenon (Fig. 3). Spontaneous inpatient responses between KMG and EMG did not behave uniformly (i.e., in some patients with fade in EMG, reverse fade was detected in KMG), however within-patient responses achieved with the same method remained constant over time. It is unlikely that the spontaneous/reverse fade in this context is actually produced in neuromuscular junction, because that would drift mechanic and electromyographic responses to same direction. On that account the spontaneous fade is more likely caused by the differences/inaccuracies elsewhere, perhaps between neuromuscular sensors.
As the KMG gave higher TOF ratios than EMG, T1 % seemed to behave opposite way. After the very beginning of recovery KMG T1 % started to lag behind EMG T1 %. Single twitch measurements are, however, only seldom used in modern practice making the finding clinically insignificant.
In daily clinical practice we have seen that the automatic algorithm used by NMT module has sometimes failed to achieve proper supramaximal nerve stimulation. This may lead to irrational/aberrant responses especially if the stimulation current is too high. This is mostly caused by muscle contractions near the stimulating electrodes, causing additional movement to the recording site thus leading to irrelevant TOF responses. This caused one dropout in our study, raising the question, whether the method that the NMT module uses to ensure supramaximal stimulation is reliable enough for scientific purposes.
As a limitation of the study, TOF responses were not recorded simultaneously. In our ipsilateral setting this was impossible, because a proper (20 s) interval between stimulations was needed to ensure full recovery of the neuromuscular junction before the next stimulation. This interval caused bias that is related to the speed of recovery and time interval between stimulations. However, in most of the cases TOF ratio recovered very gradually, minimizing the impact of the recovery bias.
In conclusion, we found significant difference in TOF ratios between EMG and KMG, and these sensors can not be used interchangeably with same reference values. This confirms the earlier observations. Because of the aforementioned discrepancies between different sensors, further validation of KMG with direct clinical endpoints is recommended. In the meanwhile we suggest aiming TOF-ratios well above 90 % with KMG before the removal of endotracheal tube.
References
Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789–808.
Eriksson LI, Sundman E, Olsson R, Nilsson L, Witt H, Ekberg O, Kuylenstierna R. Functional assessment of the pharynx at rest and during swallowing in partially paralyzed humans: simultaneous videomanometry and mechanomyography of awake human volunteers. Anesthesiology. 1997;87:1035–43.
Miller. Neuromuscular monitoring. In: Miller’s anesthesia. 7th ed. Chapter 47.
Eriksson LI, Lennmarken C, Wyon N, Johnson A. Attenuated ventilatory response to hypoxaemia at vecuronium-induced partial neuromuscular block. Acta Anaesthesiol Scand. 1992;36:710–5.
Eriksson LI, Sato M, Severinghaus JW. Effect of a vecuroniuminduced partial neuromuscular block on hypoxic ventilator response. Anesthesiology. 1993;78:693–9.
Eriksson LI. Reduced hypoxic chemosensitivity in partially paralysed man. A new property of muscle relaxants? Acta Anaesthesiol Scand. 1996;40:520–3.
Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans. Pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology. 2000;92:977–84.
Carter JA, Arnold R, Yate PM, Flynn PJ. Assessment of the Datex Relaxograph during anaesthesia and atracurium-induced neuromuscular blockade. Br J Anaesth. 1986;58:1447–52.
Motamed C, Kirov K, Combes X, Duvaldestin P. Comparison between the Datex-Ohmeda M-NMT module and a forcedisplacement transducer for monitoring neuromuscular blockade. Eur J Anaesth. 2003;20:467–9.
Dahaba AA, von Klobucar F, Rehak PH, List WF. The neuromuscular transmission module versus the relaxometer mechanomyograph for neuromuscular block monitoring. Anesth Analg. 2002;94:591–6.
Stewart PA, Freelander N, Liang S, Heller G, Phillips S. Comparison of electromyography and kinemyography during recovery from non-depolarising neuromuscular blockade. Anaesth Intensive Care. 2014;42(3):378–84.
Gaffar EA, Fattah SA, Atef HM, Omera MA, Abdel-Aziz MA. Kinemyography (KMG) versus Electromyography (EMG) neuromuscular monitoring in pediatric patients receiving cisatracurium during general anesthesia. Egypt J Anaesth. 2013;29:247–53.
NMT Appliguide. GE-Healthcare OY, Helsinki, Finland.
Schnider TW, Minto CF, Gambus PL, Andresen C. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–82.
Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil II. Model application. Anesthesiology. 1997;86:24–33.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Claudius C, Skovgaard LT, Viby-Mogensen J. Arm-to-arm variation when evaluating neuromuscular block: an analysis of the precision and the bias and agreement between arms when using mechanomyography or acceleromyography. Br J Anaesth. 2010;105(3):310–7.
Cammu G, De Witte J, De Veylder J, Byttebier G, Vandeput D, Foubert L, Vandenbroucke G, Deloof T. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg. 2006;102:426–9.
Ueda N, Muteki T, Tsuda H, Inoue S, Nishina H. Is the diagnosis of significant residual neuromuscular blockade improved by using double-burst nerve stimulation? Eur J Anaesthiol. 1991;8:213–8.
Viby-Mogensen J, Jensen NH, Engbæk J, et al. Tactile and visual evaluation of the response to train-of-four nerve stimulation. Anesthesiology. 1985;63:440.
Acknowledgments
Authors do not have a financial relationship with the organization that sponsored the research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salminen, J., van Gils, M., Paloheimo, M. et al. Comparison of train-of-four ratios measured with Datex-Ohmeda’s M-NMT MechanoSensor™ and M-NMT ElectroSensor™. J Clin Monit Comput 30, 295–300 (2016). https://doi.org/10.1007/s10877-015-9717-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9717-4