Abstract
Objective
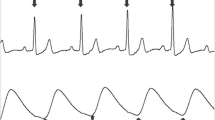
It has been widely appreciated that ventilation-induced variations in systolic blood pressure during mechanical ventilation correlate with changes in intravascular volume. The present study assessed whether alterations in volume status likewise can be detected with noninvasive monitoring (ear plethysmograph) in non-intubated subjects (awake volunteers).
Methods
Eight healthy adults were monitored with EKG, noninvasive blood pressure, an unfiltered ear plethysmograph, and a respiratory force transduction belt before (PRE) and after (POST) withdrawal of 450 ml of blood from an antecubital vein. Spectral-domain analysis was used to determine the peak ventilatory frequency and the power of the associated variation in the ear plethysmographic tracing; Interphase differences in the respiration-induced plethysmographic variations were assessed by Wilcoxon signed rank test. In addition, the changes in the ear plethysmographic tracing were compared to changes in heart rate and blood pressure.
Results
There was a significant increase in respiratory-associated oscillations at the respiratory frequency between the PRE and POST phases (p = 0.012). These changes were detected despite lack of changes in heart rate or blood pressure.
Conclusions
Respiration-induced changes of the ear plethysmographic waveform during spontaneous ventilation increase significantly as a consequence of withdrawal of approximately one unit of blood in healthy volunteers.
Similar content being viewed by others
References
Bilchick KC, Wise RA. Paradoxical physical findings described by kussmaul: pulsus paradoxus, kussmaul’s sign. Lancet 2002; 359: 1940–2
Jardin F, Farcot J, Gueret P, Prost J, Ozier Y, Bourdarias J. Cyclic changes in arterial pulse during respiratory support. Circulation 1983; 68: 266–74
Khan F, Davidson NC, Littleford RC, Litchfield SJ, Struthers AD, Belch JJ. Cutaneous vascular responses to acetylcholine are mediated by a prostanoid-dependent mechanism in man. Vasc Med 1997; 2: 82–6
Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005; 103: 419–28
Morgan B, Crawford W, Guntheroth W. The hemodynamic effects of changes in blood volume during intermittent positive-pressure ventilation. Anesthesiology 1969; 30: 297–305
Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology 1987; 67: 498–502
Pizov R, Ya’ari Y, Perel A. Systolic pressure variation is greater during hemorrhage than during sodium nitroprusside-induced hypotension in ventilated dogs. Anesth Analg 1988; 67: 170–4
Szold A, Pizov R, Segal E, Perel A. The effect of tidal volume and intravascular volume state on systolic pressure variation in ventilated dogs. Intensive Care Med 1989; 15: 368–71
Pizov R, Segal E, Kaplan L, Floman Y, Perel A. The use of systolic pressure variation in hemodynamic monitoring during deliberate hypotension in spine surgery. J Clin Anesth 1990; 2: 96–100
Connors A, Speroff T, Dawson N. The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA 1996; 276: 889–97
Marik PE. The systolic blood pressure variation as an indicator of pulmonary capillary wedge pressure in ventilated patients. Anaesth Intensive Care 1993; 21: 405–8
Ornstein E, Eidelman LA, Drenger B, Elami A, Pizov R. Systolic pressure variation predicts the response to acute blood loss. J Clin Anesth 1998; 10: 137–40
Perel A. Cardiovascular assessment by pressure waveform analysis. ASA Annual Refresher Course Lecture 1991:264
Preisman S, Kogan S, Berkenstadt H, Perel A. Predicting fluid responsiveness in patients undergoing cardiac surgery: functional haemodynamic parameters including the respiratory systolic variation test and static preload indicators. Br J Anaesth 2005; 95: 746–55
Rooke GA, Schwid HA, Shapira Y. The effect of graded hemorrhage and intravascular volume replacement on systolic pressure variation in humans during mechanical and spontaneous ventilation. Anesth Analg 1995; 80: 925–32
Yamakage M, Itoh T, Iwasaki S, Jeong S-W, Namiki A. Variation of “Pulse amplitude” Measured by a pulse oximeter may help predict intravascular volume Can J Anaesth 2005; 52: 207–8
Cohn JN, Pinkerson AL, Tristani FE. Mechanism of pulsus paradoxus in clinical shock. J Clin Invest 1967; 46: 1744–55
Dorlas JC, Nijboer JA. Photo-electric plethysmography as a monitoring device in anaesthesia. Application and interpretation. Br J Anaesth 1985; 57: 524–30
Johansson A, Oberg PA. Estimation of respiratory volumes from the photoplethysmographic signal. Part i: experimental results. Med Biol Eng Comput 1999; 37: 42–7
Lherm T, Chevalier T, Troche G, Souron V, Samaha T, Zazzo J. Correlation between plethysmography curve variation (dpleth), pulmonary capillary wedge pressure (pcwp) in mechanically ventilated patients. Br J Anaesth 1995; Suppl. 1: 41
Partridge BL. Use of pulse oximetry as a noninvasive indicator of intravascular volume status. J Clin Monit 1987; 3: 263–8
Shamir M, Eidelman LA, Floman Y, Kaplan L, Pizov R. Pulse oximetry plethysmographic waveform during changes in blood volume. Br J Anaesth 1999; 82: 178–81
Golparvar M, Naddafnia H, Saghaei M. Evaluating the relationship between arterial blood pressure changes and indices of pulse oximetric plethysmography. Anesth Analg 2002; 95: 1686–90
Awad A, Ghobashy MA, Ouda W, Stout RG, Silverman DG, Shelley KH. Different responses of ear and finger pulse oximeter wave form to cold pressor test. Anesth Analg 2001; 92: 1483–6
Hertzman AB. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am J Physiol 1938; 124: 328–40
Shelley KH, Jablonka DH, Awad AA, Stout RG, Rezkanna H, Silverman DG. What is the best site for measuring the effect of ventilation on the pulse oximeter waveform? Anesth Analg 2006; 103: 372–7
Shelley KH, Awad AA, Stout RG, Silverman DG. The use of joint time frequency analysis to quantify the effect of ventilation on the pulse oximeter waveform. J Clin Monit Comput 2006; 20: 81–7
Shelley KH, Tamai D, Jablonka D, Gesquiere M, Stout RG, Silverman DG. The effect of venous pulsation on the forehead pulse oximeter wave form as a possible source of error in spo2 calculation. Anesth Analg 2005; 100: 743–7
The acute respiratory distress syndrome network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8
Morelot-Panzini C, Lefort Y, Derenne JP, Similowski T. Simplified method to measure respiratory-related changes in arterial pulse pressure in patients receiving mechanical ventilation. Chest 2003; 124: 665–70
Bendjelid K, Suter PM, Romand JA. The respiratory change in preejection period: a new method to predict fluid responsiveness. J Appl Physiol 2004; 96: 337–42
Reuter DA, Kirchner A, Felbinger TW, Weis FC, Kilger E, Lamm P, Goetz AE. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med 2003; 31: 1399–404
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded only with departmental funds.
Presented, in part, at the annual meeting of the American Society of Anesthesiologists, October, 2004.
Implications Statement: Quantification of respiration-induced changes of an ear plethysmographic signal provided an indication of volume loss after withdrawal of 450 ml of blood in healthy, non-intubated volunteers.
Gesquiere MJ, Awad AA, Silverman DG, Stout RG, Jablonka DH, Silverman TJ, Shelley KH. Impact of Withdrawal of 450 ml of Blood on Respiration-Induced Oscillations of the Ear Plethysmographic Waveform.
Rights and permissions
About this article
Cite this article
Gesquiere, M.J., Awad, A.A., Silverman, D.G. et al. Impact of Withdrawal of 450 ml of Blood on Respiration-Induced Oscillations of the Ear Plethysmographic Waveform. J Clin Monit Comput 21, 277–282 (2007). https://doi.org/10.1007/s10877-007-9085-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-007-9085-9