Abstract
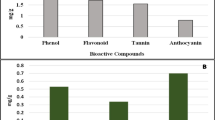
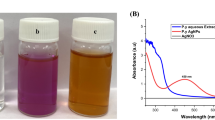
This study reports on the bioactive potential of silver nanoparticle synthesized from seaweed, Cladophora fascicularis aqueous extract. C. fascicularis assisted silver nanoparticles (Cf-AgNPs) was characterized by UV–visible spectroscopy, XRD, FT-IR, FESEM, TEM, and EDX analysis. The Cf-AgNPs exhibited a maximum absorption peak at 427 nm. The carboxyl (–C=O) and amine (N–H) groups of C. fascicularis extract is answerable for the synthesis of Cf-AgNPs. The microscopic analysis confirmed that the synthesized Cf-AgNPs are spherical in shape with an average size of 45 nm. The Cf-AgNPs exhibited a dose depended antibacterial activity against Aeromonas hydrophila. Noteworthy bacterial inhibition (19 mm) was observed at 150 µg/ml, whereas, the lowest activity (10 mm) was recorded at 25 µg/ml. Similarly, Cf-AgNPs significantly (P < 0.05) enhanced the protein leakage in A. hydrophila by increasing the membrane permeability. The considerable amount of ROS activity was noticed in the test bacterium A. hydrophila when treated with 150 µg/ml of Cf-AgNPs. The toxicity investigation results revealed that the synthesized Cf-AgNPs were not acutely toxic to Artemia nauplii even at a higher concentration (150 μg/ml). These results underline that Cf-AgNPs effectively inhibited the growth of A. hydrophila and showed the non-toxic effect with outstanding biocompatibility.












Similar content being viewed by others
References
FAO The State of World Fisheries and Aquaculture 2016 (SOFIA): contributing to food security and nutrition for all (Food and Agriculture Organization, Rome, 2016), p. 200.
FAO State of World Aquaculture (Food and Agriculture Organization of the United Nations, Rome, 2008), p. 176.
S. Jeremic, D. Jakic-Dimic, and L. Veljovic (2003). Acta. Veterinaria.53, 399–410.
P. H. Klesius, J. J. Evans, and C. A. Shoemaker (2007). Fish Shellfish Immun.22, 443–450.
D. V. Lightner (2011). J Invertebr. Pathol.106, 110–130.
I. Zorrilla, M. Chabrillon, S. Arijo, P. Diaz-Rosales, and E. Martinez-Manzanares (2003). Aquaculture.218, 11–20.
J. M. Janda and S. L. Abbott (2010). Clin. Microbiol. Rev.23, 35–73.
S. L. Angka, T. J. Lam, and Y. M. Sin (1995). Aquaculture.130, 103–112.
H. Neethu, K.T. Tincy, and A.N. Jayakumaran (2013). ISRN Nanotechnol. https://doi.org/10.1155/2013/792105.
P. Raji, Antony V. Samrot, D. Keerthanam, and S. Karishma (2019). J. Clust. Sci.30, 881.
A. Maniraj, M. Kannan, K. Rajarathinam, S. Vivekanandhan, and S. Muthuramkumar (2019).J.Clust. Sci.(in press).
K. H. Cho, J. E. Park, T. Osaka, and S. G. Park (2005). Electrochimica Acta.51, 956–960.
E. M. Petrus, S. Tinakumari, L. C. Chai, A. Ubong, R. Tunung, N. Elexson, L. F. Chai, and R. Son (2011). Int. Food. Res J.18, 55–66.
W. R. Li, X. B. Xie, Q. S. Shi, H. Y. Zeng, Y. S. Ou-Yang, and Y. B. Chen (2010). Appl. Microbiol. Biotechnol.85, 1115–1122.
J. S. Kim, E. Kuk, K. N. Yu, J. H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C. Y. Hwang, Y. K. Kim, Y. S. Lee, D. H. Jeong, and M. H. Cho (2007). J. Nanomed.3, 95–101.
B. Sarkar, A. Mahanty, S. P. Netam, S. Mishra, N. Pradhan, and M. Samanta (2012). Int. J. Nanomater. Biostruct.2, 70–74.
P. Kalimuthu and S. John (2008). J. Electroanal. Chem.617, 164–170.
K. Kanagamani, P. Muthukrishnan, M. Ilayaraja, J. Kumar, K. Shankar, and A. Kathiresan (2017). J. Photochem. Photobiol A.346, 470–478.
K. Kanagamani, P. Muthukrishnan, M. Ilayaraja, K. Shankar, and A. Kathiresan (2018). J. Inorganic Organometal. Polymers Mater.28, (3), 702–710.
K. Kanagamani, P. Muthukrishnan, K. Shankar, A. Kathiresan, H. Barabadi, M. Saravanan (2019). J. Clust. Sci. https://doi.org/10.1007/s10876-019-01583-y.
S. Palanisamy, R. Anjali, M. Vinosha, M. Reka, S. Selvakumar, P. Boomi, K. Anand, N.M. Prabhu, S.S.N. Somasundaram, S. You (2019).Process Biochem. (in press).
A. Ravichandran, P. Subramanian, V. Manoharan, T. Muthu, R. Periyannan, M. Thangapandi, K. Ponnuchamy, B. Pandi, and P. N. Marimuthu (2018). J. Photochem. Photobiol B.185, 117–125.
S. Palanisamy, P. Rajasekar, G. Vijayaprasath, G. Ravi, R. Manikandan, and N. M. Prabhu (2017). Mater. Lett.189, 196–200.
M. Hjelm, O. Bergh, A. Riaza, J. Nielsen, J. Melchiorsen, S. Jensen, H. Duncan, P. Ahrens, H. Birkbeck, and L. Gram (2004). Syst. Appl. Microbiol.27, 360–371.
K. Chauhan, R. Sharma, R. Dharela, G. S. Chauhan, and R. K. Singhal (2016). RSC Adv.79, 2016.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall (1951). J. Biol. Chem.193, 265–275.
I. S. Kye, Y. S. Jeon, J. K. No, and A. Kim (1999). J. Korean Gerontol. Nurs.9, 10–17.
C. Arulvasu, S. M. Jennifer, D. Prabhu, and D. Chandhirasekar (2014). Sci World J2014, 256919.
H. Jiang, K. S. Moon, Z. Zhang, S. Pothukuchi, and C. Wong (2006). J Nanopart.8, 117–124.
S. Muthukrishnan, S. Bhakya, T. S. Kumar, and M. Rao (2015). Ind. Crops Prod.63, 119–124.
N. Kanipandian, S. Kannan, R. Ramesh, P. Subramanian, and R. Thirumurugan (2014). Mater. Res. Bull.49, 494–502.
M. Ahmad, F. Mohammed, F. Maqbool, A. Azamand, and S. Iqbal (2003). Sarhad J. Agric.19, 347–351.
S. Link and M. A. El-Sayed (1999). J. Phys. Chem. B.103, 8410–8426.
K. Chauhan, R. Sharma, R. Dharela, G. S. Chauhan, and R. K. Singhal (2016). RSC Adv.6, 75453–75464.
V. Sridhara, K. Pratima, G. Krishnamurthy, and B. Sreekanth (2013). Asian J Pharm Clin Res.6, 53–57.
R. Swarup and D. T. Kumar (2014). Nanosci. Nanotechnol. Lett.6, 181.
A. Ghadimi, R. Saidur and H.S.C Metselaar (2011). Int J Heat Mass Transf. 54, 4051-4068.
R. Augustine, N. Kalarikkal, and S. Thomas (2014). Appl. Nanosci.4, 809–818.
S. Patil, J. Fernandes, R. Tangasali, and I. Furtado (2014). J Clust Sci.25, 423–433.
R. Bhat, S. Ganachari, and R. Deshpande (2013). J Clust Sci.24, 107.
H. Alishah, S. P. Seyedi, S. Y. Ebrahimipour, and S. Esmaeili-Mahani (2016). J. Clust. Sci.27, 421–429.
S. K. Das, M. M. R. Khan, A. K. Guha, A. R. Das, and A. B. Mandal (2012). Bioresour Technol.124, 495–499.
M. Moyo, M. Gomba, and T. Nharingo (2015). Int. J. Ind. Chem.6, 329–338.
K. Kathiresan, S. Manivannan, M. A. Nabeal, and B. Dhivya (2009). Coll. Surf. B.71, 133–137.
A. Nabikhan, K. Kandasamy, A. Raj, and N. M. Alikunh (2010). Coll. Surf. B Biointerfaces.79, 488–493.
A. Sankaranarayanan, G. Munivel, G. Karunakaran, S. Kadaikunnan, N. S. Alharbi, J. M. Khaled, and D. Kuznetsov (2017). J. Clust. Sci.28, 995–1008.
A. Shahzad, H. Saeed, M. Iqtedar, S. Z. Hussain, A. Kaleem, R. Abdullah, S. Sharif, S. Naz, F. Saleem, A. Aihetasham, and A. Chaudhary (2019). J. Nanomater2019, 5168698.
S. Pal, Y. K. Tak, and J. M. Song (2007). Appl. Environ. Microbiol. Mar.73, 1712–1720.
S. Gurunathana (2019). Arab. J. Chem.12, 168–180.
S. H. Kim, H. S. Lee, D. S. Ruy, S. J. Choi, and D. S. Lee (2011). Korean J. Microbiol. Biotechnol.39, 77–85.
D. K. Tiwari, J. Behari, and P. Sen (2008). Currt. Sci.95, 647–655.
A. Nel, T. Xia, L. Madler, and N. Li (2006). Science.311, 622–627.
H. L. Su, C. C. Chou, D. J. Hung, S. H. Lin, I. C. Pao, J. H. Lin, F. L. Huang, R. X. Dong, and J. J. Lin (2009). Biomaterials.30, 5979–5987.
Y. Matsumura, K. Yoshikata, S. Kunisaki, and T. Tsuchido (2003). Appl. Environ. Microbiol.69, 4278–4281.
S. R. K. Pandian, V. Deepak, K. Kalishwaralal, P. Viswanathan, and S. Gurunathan (2010). Braz. J. Microbiol.41, 805–809.
I. Maliszewska and Z. Sadowski (2009). J. Phys Conf Ser.1, 146–151.
C. Dipankar and S. Murugan (2012). Colloids Surf B.98, 112–119.
X. P. Nunes, G. L. A. Maia, J. R. G. S. Almeida, F. O. Pereira, and E. O. Lima (2006). Rev Bras Farmacogn.16, 642–644.
V. Kokkali, I. Katramados, and J. D. Newman (2011). Biosensors.1, 45–49.
M. Ates, J. Daniels, Z. Arslan, and I. O. Farah (2013). Environ. Monit. Assess.185, 3339–3348.
M. Latha, M. Priyanka, P. Rajasekar, R. Manikandan, and N. M. Prabhu (2016). Microb. Pathog.93, 88–94.
Acknowledgment
The authors are thankful to RUSA scheme Phase 2.0 Grant [F-24-51/2014–U, Policy (TNMulti-Gen), Dept of Edn, Govt. of India. Dt. 09.10.2018] for their financial support. The author would like to thank the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1A6A1A03023584) for the support to complete this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajasekar, P., Palanisamy, S., Anjali, R. et al. Cladophora fascicularis Mediated Silver Nanoparticles: Assessment of Their Antibacterial Activity Against Aeromonas hydrophila. J Clust Sci 31, 673–683 (2020). https://doi.org/10.1007/s10876-019-01674-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01674-w