Abstract
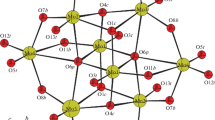
Two novel phosphomolybdate derivatives, (H2en)(Hen)2(H2P2Mo5O23)·5H2O (1) and [Cu(phen)(en)][Cu(phen)(en)(H2O)]2[PMo VI8 Mo V4 O40{Cu(phen)}2]2·6H2O (2) (en = ethylenediamine, phen = 1,10′-phenanthroline), were synthesized under hydrothermal technique and characterized by crystal X-ray diffraction analysis, elemental analysis, IR and TGA. Compound 1 is constructed from a [H2P2Mo5O23]4− polyoxoanion and one (H2en)2+ cation and two (Hen)+ cations and is a typical Strandberg-type hepteropoly compound. The interaction force between the components are electrostatic force and hydrogen bonds which involve polyoxoanions, water molecules and ethylenediammonium cations. Compound 2 consists of one [Cu(phen)(en)]2+ and two [Cu(phen)(en)(H2O)]2+ cations and two [PMo VI8 Mo V4 O40{Cu(phen)}2]3− polyoxoanions. The reduced Keggin polyoxoanion [PMo VI8 Mo V4 O40]7− of 2 is capped by two divalent Cu atoms through four bridging oxo groups on two opposite {Mo4O4} faces. The results reveal that the pH plays a significant role in promoting the diversity of structural motifs. The effect of en is not only to ensure the required amounts of ligands for the reaction process, but also to control the pH. In the synthetic process, the control of the reaction conditions plays a crucial role in constructing different structures.




Similar content being viewed by others
References
M. T. Pope and A. Müller (1991). Angew. Chem. Int. Ed. Engl. 30, 34.
C. L. Hill (1998). Chem. Rev. 98, 1.
P. J. Hagrman, D. Hagrman, and J. Zubieta (1999). Angew. Chem. Int. Ed. 38, 2638.
H. Q. Tan, Y. G. Li, Z. M. Zhang, C. Qin, X. L. Wang, E. B. Wang, and Z. M. Su (2007). J. Am. Chem. Soc. 129, 10066.
J. P. Wang, H. X. Ma, L. C. Zhang, W. S. You, and Z. M. Zhu (2014). Dalton Trans. 43, 17172–17176.
Z. L. Li, Y. Wang, L. C. Zhang, J. P. Wang, W. S. You, and Z. M. Zhu (2014). Dalton Trans. 43, 5840–5846.
H. X. Ma, J. Du, Z. M. Zhu, T. Lu, F. Su, and L. C. Zhang (2016). Dalton Trans. 45, 1631–1637.
S. C. Sun, X. Liu, L. Yang, H. Tan, and E. B. Wang (2016). Eur. J. Inorg. Chem. 2016, 4179–4184.
Y. Ammari, E. Dhahri, M. Rzaigui, E. K. Hlil, and S. Abid (2016). J. Clust. Sci. 27, 1213–1227.
C. Dey, T. Kundu, and R. Banerjee (2012). Chem Commun. 4848, 266–268.
I. Nagazi and A. Haddad (2013). J. Clust. Sci. 24, 145–155.
L. Xu, H. Zhang, E. Wang, A. Wu, and Z. Li (2002). Mater. Lett. 54, 452–457.
J. X. Meng, Y. G. Li, X. L. Wang, and E. B. Wang (2009). J. Coord. Chem. 62, 2283–2289.
X. G. Cao, L. W. He, B. Z. Lin, and Z. J. Xiao (2010). J. Chem. Crystallogr. 40, 443–447.
S. Upreti and A. Ramanan (2006). Crys. Growth Des. 6, 2066–2071.
Y. Wang, L. C. Zhang, Z. M. Zhu, N. Li, A. F. Deng, and S. Y. Zheng (2011). Trans. Met. Chem. 36, 261–267.
S. R. Jin, L. M. Zhang, S. Z. Liu, L. Bo, and X. G. Meng (2008). J. Wuhan Univ. Technol.-Mater. Sci. Ed. 23, 407–410.
J. Lu, Y. Xu, N. K. Goh, and L. S. Chia (1998). Chem. Commun. 24, 2733–2734.
E. Burkholder and J. Zubieta (2001). Chem. Commun. 20, 2056–2057.
M. Bartholom, H. Chueng, S. Pellizzeri, K. Ellis-Guardiola, S. Jones, and J. Zubieta (2012). Inorg. Chim. Acta 389, 90–98.
M. Carraro, A. Sartorel, G. Scorrano, C. Maccato, M. H. Dickman, U. Kortz, and M. Bonchio (2008). Angew. Chem. Int. Ed. 47, 7275–7279.
M. P. Lowe, J. C. Lockhart, W. Clegg, and K. A. Fraser (1994). Angew. Chem. Int. Ed. 33, 451–454.
J. Niu, J. Ma, J. Zhao, P. Ma, and J. Wang (2011). Inorg. Chem. Commun. 14, 474–477.
K. Yu, B.-B. Zhou, Y. Yu, Z.-H. Su, H. Wang, C. Wang, and C. Wang (2012). Dalton Trans. 41, 10014–10020.
Q. Chen and C. L. Hill (1996). Inorg. Chem. 35, 2403.
F. Y. Li, L. Xu, Y. G. Wei, and E. B. Wang (2005). Inorg. Chem. Commun. 8, 263.
Y. G. Li, E. B. Wang, and S. T. Wang (2002). J. Mol. Struct. 611, 185.
J. X. Meng, Y. Lu, Y. G. Li, H. Fu, and E. B. Wang (2011). Cryst. Eng. Commun. 13, 2479–2486.
A. Müller, C. Beugholt, P. Kögerler, H. Bögge, S. Bud’ko, and M. Luban (2000). Inorg. Chem. 39, 5176–5177.
M. Yuan, Y. G. Li, E. B. Wang, C. G. Tian, N. H. Hu, and H. Q. Jia (2003). Inorg. Chem. 42, 3670–3676.
Y. P. Bai, Y. G. Li, E. B. Wang, X. L. Wang, Y. Lu, and L. Xu (2005). J. Mol. Struct. 752, 54–59.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann (2009). J. Appl. Cryst. 42, 339–341.
G. M. Sheldrick (2015). Acta. Cryst. A71, 3–8.
G. M. Sheldrick (2015). Acta. Cryst. C71, 3–8.
Z. Z. Lin, H. H. Zhang, C. C. Huang, and R. Q. Sun (2002). Chinese J. Struct. Chem. 21, 42–45.
J. W. Zhao, Y. Y. Li, Y. H. Wang, D. Y. Shi, J. Luo, and L. J. Chen (2013). Russ. J. Coord. Chem. 39, 519–523.
R. Strandberg (1973). Acta. Chem. Scand. 27, 1004–1018.
T. Akutagawa, D. Endo, H. Imai, S. Noro, L. Cronin, and T. Nakamura (2006). Inorg. Chem. 45, 8628–8637.
F. Zocchi (2006). Chem. Phys. Let. 421, 277–280.
Funding
This study was supported by Jilin Agricultural Science and Technology University the Science Foundation (No. 2015246).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, FX., Chen, YG., Yang, HY. et al. The pH-Controlled Hydrothermal Synthesis and Crystal Structure of Two Novel Phosphomolybdate Derivatives. J Clust Sci 30, 123–129 (2019). https://doi.org/10.1007/s10876-018-1468-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1468-1