Abstract
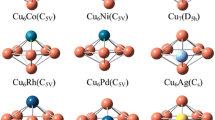
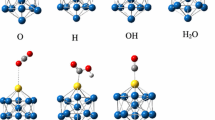
Density functional theory calculations were carried out to investigate Cu12TM (TM = Co, Rh, Ir, Ni, Pd, Pt, Ag, Au) bimetallic metal catalysts for the mechanism of reverse water–gas shift (RWGS) reaction. The three possible reaction pathways relevant to the RWGS reaction are explored, including the CO2 dissociation, carboxyl, and formate mechanisms. Our results indicate that the RWGS reaction prefers to follow the CO2 dissociation mechanism on Cu12TM surfaces. A detailed potential energy diagram of the kinetically favored mechanism is presented that shows that the RDS of reaction are the formation of H2O and carboxyl (HOCO), formate (HCOO) dissociation, respectively. And, Cu12TM (TM = Co, Pt) are lower than other catalysts from the energy barrier of elementary step. Moreover, the catalytic behavior of a Cu12TM cluster is changed significantly due to the modifiers, via the electron transfer from TM to Cu-based cluster, and the activation barrier decreases with doped TM. The turnover frequency of the Cu12Co is the highest value, which thus is more efficiency catalyst to RWGS reaction. To gain insights into the synergistic effect in catalytic activity of the Cu12TM bimetallic cluster, a projected density of states analysis has been performed. Our works will be important for predicting the energetic trends and designing a better catalyst of RWGS reaction.







Similar content being viewed by others
References
M. S. Wainwright and D. L. Trimm (1995). Catal. Today. 23, 29.
G. Momen, G. Hermosillaa, A. Michaua, M. Ponsc, M. Firdaoussc, and K. Hassounia (2009). Int. J. Hydrogen Energy. 34, 3799.
S. H. Hakim, C. Sener, A. C. Alba-Rubio, T. M. Gostanian, B. J. O’Neill, F. H. Ribeiro, J. T. Miller, and J. A. Dumesic (2015). J. Catal. 328, 75.
C. S. Chena, W. H. Cheng, and S. S. Lin (2003). Appl. Catal. A-Gen. 238, 55–67.
R. G. Zhang, B. J. Wang, H. Y. Liu, and L. X. Ling (2011). J. Phys. Chem. C 115, 19811.
Y. F. Zhao, Y. Yang, C. Mims, C. H. F. Peden, J. Li, and D. H. Mei (2011). J. Catal. 281, 199.
M. S. Spencer (1995). Catal. Lett. 32, 9.
K. Shin, D. H. Kim, S. C. Yeo, and H. M. Lee (2012). Catal. Today. 185, 94.
J. Knudsen, A. U. Nilekar, R. T. Vang, J. Schnadt, E. L. Kunkes, J. A. Dumesic, M. Mavrikakis, and F. Besenbacher (2007). J. Am. Chem. Soc. 129, 6485.
J. Nakamura, J. M. Campbell, and C. T. Campbell (1990). J. Chem. Soc. Faraday Trans. 26, 2725.
X. W. Nie, H. Z. Wang, M. J. Janik, X. W. Guo, and C. S. Song (2016). J. Phys. Chem. C 120, 9364.
M. J. L. Ginés, A. J. Marchi, and C. R. Apesteguía (1997). Appl. Catal. A-Gen. 154, 155.
S. I. Fujita, M. Usui, and N. Takezawa (1992). J Catal. 134, 1220.
K. H. Ernst, C. T. Campbell, and G. Moretti (1992). J. Catal. 134, 66.
C. S. Chen, W. H. Cheng, and S. S. Lin (2000). Catal. Lett. 68, 45.
C. S. Chen and W. H. Cheng (2002). Catal. Lett. 83, 121–126.
G. C. Wang, L. Jiang, X. Y. Pang, Z. S. Cai, Y. M. Pan, X. Z. Zhao, Y. Morikawa, and J. Nakamura (2003). Surf. Sci. 543, 118.
G. C. Wang, L. Jiang, Y. H. Zhou, Z. S. Cai, Y. M. Pan, X. Z. Zhao, Y. W. Li, Y. H. Sun, B. Zhong, X. Y. Pang, W. Huang, and K. C. Xie (2003). J. Mol. Struct. 634, 23.
Y. A. Daza and J. N. Kuhn (2016). RSC Adv. 6, 49675.
D. H. Mei, L. J. Xu, and G. Henkelman (2008). J. Catal. 258, 44.
L. Dietz, S. Piccinin, and M. Maestri (2015). J. Phys. Chem. C 119, 4959.
N. Ishito, K. Hara, K. Nakajima, and A. Fukuokaa (2016). J. Energy Chem. 25, 306.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, and J. R. Cheeseman Gaussian 03 (Revision C02) (Gaussian Inc, Pittsburgh, 2009).
W. R. Wadt and P. J. Hay (1985). J. Chem. Phys. 82, 284.
P. J. Hay and W. R. Wadt (1985). J. Chem. Phys. 82, 299.
J. P. Perdew, K. Burke, and M. Ernzerhof (1996). Phys. Rev. Lett. 77, 3865.
C. Peng, P. Y. Ayala, and S. H. Bernhard (1996). J. Comput. Chem. 17, 49.
K. P. Huber and G. Herzberg Molecular Spectra and Molecular Structure, vol. 4 (Van Nostrand Reinhold, New York, 1979).
V. L. Mazalova and A. V. Soldatov (2009). J. Phys. Chem. C 113, 9086.
A. A. Gokhale, J. A. Dumesic, and M. Mavrikakis (2008). J. Am. Chem. Soc. 130, 1402.
S. Kattel, B. H. Yan, Y. X. Yang, J. G. G. Chen, and P. Liu (2016). J. Am. Chem. Soc. 138, 12440.
T. Fujitani, Y. Choi, M. Sano, Y. Kushida, and J. Nakamura (2000). J. Phys. Chem. B 104, 1235.
A. Sotiropoulos, P. K. Milligan, B. C. C. Cowie, and M. Kadodwala (2000). Surf. Sci. 444, 52.
F. Solymosi (1991). J. Mol. Catal. 65, 337.
S. G. Wang, X. Y. Liao, D. B. Cao, C. F. Huo, Y. W. Li, J. Wang, and H. Jiao (2007). J. Phys. Chem. C 111, 16934.
L. Barrio, P. Liu, J. A. Rodriguez, J. M. Campos-Martin, and J. L. G. Fierro (2006). J. Chem. Phys. 125, 164715.
Y. X. Yang, J. Evans, J. A. Rodriguez, M. G. White, and P. Liu (2010). Phys. Chem. Chem. Phys. 12, 9909.
B. Hammer and J. K. Norskov (1995). Nature 376, 238.
C. Amatore and A. Jutand (1999). J. Org. Chem. 576, 254.
S. A. Kozuch (2012). Comput. Mol. Sci. 2, 795.
B. Hammer and J. K. Nørskov (1995). Surf. Sci. 343, 211.
B. Hammer and J. K. Nørskov (2000). Adv. Catal. 45, 71.
C. Liu and P. Liu (2015). ACS Catal. 5, 1004.
Acknowledgements
This work was financially supported by the “1331” project of Shanxi Province, High School 131 Leading Talent Project of Shanxi, Graduate student innovation project of Shanxi Normal University, Undergraduate Training Programs for Innovation and Entrepreneurship of Shanxi Province (Grant No. 105088, 2015537, WL2015CXCY-SJ-01) and Shanxi Normal University (WL2015CXCY-YJ-18), Teaching Reform Project of Shanxi Normal University (WL2015 JGXM-YJ-13), Shanxi Normal University graduate student science and technology innovation project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Q., Guo, L. Mechanism of the Reverse Water–Gas Shift Reaction Catalyzed by Cu12TM Bimetallic Nanocluster: A Density Functional Theory Study. J Clust Sci 29, 867–877 (2018). https://doi.org/10.1007/s10876-018-1346-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-018-1346-x