Abstract
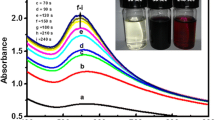
Gold nanoparticles (AuNPs) of 30–40 nm in size has been prepared using A. hirsutus leaves extract as reducing agent for Au3+ ions under microwave irradiation from 60 to 360 s. These biocapped AuNPs were effectively conjugated with activated folic acid (FA, receptor) and chlorambucil (CHL, anticancer drug) molecules. The formation of AuNPs–FA–CHL was confirmed from different characterization techniques such as XRD, UV–Visible spectra, FT-IR and TEM images. The anticancer activity of these bioconjugated AuNPs was tested against human cancer cell lines (HeLa, RKO and A549) in comparison with normal epithelial cells (Vero). Unlike AuNPs and CHL alone, AuNPs–FA–CHL showed high toxicity towards human cancer cells by significantly decreasing the percentage viability of cells. Furthermore, the amount of drug released was found to be maximum at an ideal tumor environment pH 5.3.







Similar content being viewed by others
References
M. O. Abdel, M. B. Hadeel, E. A. Sameer, A. D. Zoheir, and F. E. Mohamed (2012). Cancer Cell Int. 12, 1.
F. Jacques, D. S. Coralie, Y. Nouara, T. Jean, S. Kevin, C. Henri, and G. Nicolas (2014). ACS Chem. Neurosci. 5, 216.
P. C. Chen, C. M. Sandra, and K. O. Adegboyega (2008). Nanotechnol. Sci. Appl. 1, 45.
R. K. O’Reilly (2007). Phil. Trans. R. Soc. A 365, 2863.
A. F. Robert (2005). Nanomed. Nanotechnol. 1, 2.
S. K. Sahoo, S. Parveen, and J. J. Panda (2007). Nanomedicine 3, 20.
P. Dan, M. K. Jeffrey, H. Seungpyo, C. F. Omid, M. Rimona, and L. Robert (2007). Nature Nanotechnol. 2, 751.
C. Laura, B. Luisa, G. Felisa, A. M. Jesús, and B. Antonio (2010). Chem. Eng. J. 164, 92.
D. Raghunandan, B. Ravishankar, G. Sharanabasava, D. R. Mahesh, V. Harsoor, M. S. Yatagatti, M. Bhagawanraju, and A. Venkataraman (2011). Cancer Nanotechnol. 2, 57.
D. S. Balaji, S. Basavaraja, D. Raghunandan, D. B. Mahesh, K. P. Belawadi, and V. Abbaraju (2008). Sci. Technol. Adv. Mater. 9, 1.
J. Hongje, R. Soo-Ryoon, K. Kostas, W. H. Sang, and M. Dal-Hee (2013). Biomaterials 34, 3503.
T. Ciprian, S. Olga, O. Anamaria, D. Mircea, P. Bobe, M. Ofelia, S. Sergiu, F. Adrian, P. Emoke, A. Mihaela, K. Gabriel, C. Victor, B. N. Ioana, and I. Alexandru (2012). J. Gastrointest. Liver Dis. 21, 187.
D. G. Jacob, P. K. Bishnu, and R. Z. Eugene (2007). J. Am. Chem. Soc. 129, 11653.
D. A. Marco, I. E. Keith, and B. Shankar (2014). J. Am. Chem. Soc. 136, 5860.
B. A. Kamen and A. Capdevila (1986). Proc. Natl. Acad. Sci. 83, 5983.
B. Fadeel and A. E. Bennett (2010). Adv. Drug Deliv. Rev. 62, 362.
G. Steinberg and R. F. Borch (2001). J. Med. Chem. 44, 69.
Y. Rui, S. Wang, P. S. Low, and D. H. Thompson (1998). J. Am. Chem. Soc. 120, 11213.
P. Sunil, O. Goldie, M. Ashmi, S. Ritu, T. Mukeshchand, and S. Madhuri (2013). J. Mater. Chem. B 1, 1361.
U. Chiara, B. Daniele, L. Giada, H. Iris, P. Christine, B. Giovanni, E. U. Ronald, and K. James (2009). Part. Fiber Toxicol. 6, 1.
M. G. Madhura, K. Islam, and M. G. Sushama (1998). Biochim. et Biophys. Acta 1381, 256.
M. Paul (1996). Langmuir 12, 788.
L. Stephan and A. E. Mostafa (1999). J. Phys. Chem. B 103, 4212.
S. Yallappa, J. Manjanna, M. A. Sindhe, N. D. Satyanarayan, S. N. Pramod, and K. Nagaraja (2013). Spectrochim. Acta A 110, 108.
T. Y. Suman, S. R. Radhika, R. Ramkumar, C. Rajthilak, and P. Perumal (2014). Spectrochim. Acta A 118, 11.
S. Jain, D. G. Hirst, and J. M. Sullivan (2012). Br. J. Radiol. 85, 101.
T. Bhuvaneswari, M. Thiyagarajan, N. Geetha, and P. Venkatachalam (2014). Acta Trop. 135, 55.
T. Y. Suman, D. Elumalai, P. K. Kaleena, and R. S. R. Radhika (2013). Ind. Crops Prod. 47, 239.
Y. S. Jae, K. J. Hyeon, and S. K. Beom (2009). Process Biochem. 44, 1133.
J. Bingbing, B. B. John, and L. Bingyun (2009). Nanotechnol. Sci. Appl. 2, 21.
K. Mohamed, W. Y. Meng, H. Eliza, M. Dusica, and S. Ursula (2015). Thno 5, 357.
M. Ashmi, P. Sunil, T. Mukeshchand, J. Dhanashree, and S. Madhuri (2014). J. Mater. Chem. B 2, 698.
K. Gopinath, K. S. Venkatesh, R. Ilangovan, K. Sankaranarayanan, and A. Arumugam (2013). Ind. Crops Prod. 50, 737.
C. D. Erik, A. M. Megan, H. Xiaohua, K. Bin, and A. E. Mustafa (2011). Chem. Soc. Rev. 40, 3391.
A. D. Mubarak, N. Thajuddin, K. Jeganathan, and M. Gunasekaran (2011). Colloids Surf. B 85, 360.
M. Aradhana, K. Madhuree, P. Shipra, C. Vasvi, K. C. Gupta, and C. S. Nautiyal (2014). Bioresour. Technol. 166, 235.
B. N. Kannan and S. Natarajan (2010). Mater. Charact. 61, 1232.
C. Jingyi, S. Fusayo, J. W. Benjamin, H. Cang, J. C. Michael, Y. L. Zhi, A. Leslie, Z. Hui, B. K. Michael, L. Xingde, and X. Younan (2005). Nano Lett. 5, 473.
M. F. Jesus, C. B. Catherine, O. R. Mathis, and S. G. C. Adam (2006). Langmuir 22, 3286.
D. B. Sarah, N. Paola, S. Jo-Ann, S. David, R. E. Paul, V. Balaji, J. F. David, A. P. Jane, G. Duncan, and J. W. Nial (2010). J. Am. Chem. Soc. 132, 4678.
N. H. Dong, H. Y. Dae, M. Ho-Jin, B. L. Jung, S. B. Min, C. L. Sang, J. L. Won, S. In-Cheol, and K. Keun (2012). Biomaterials 33, 856.
A. Jaganathan, K. Murugan, C. Panneerselvam, P. Madhiyazhagan, D. Dinesh, C. Vadivalagan, A. T. Aziz, B. Chandramohan, U. Suresh, R. Rajaganesh, J. Subramaniam, M. Nicoletti, A. Higuchi, A. A. Alarfaj, M. A. Munusamy, S. Kumar, and G. Benelli (2016). Parasitol. Int. 65, (3), 276.
K. Murugana, C. Panneerselvamb, C. M. Samidossa, P. Madhiyazhagana, U. Suresha, M. Ronia, B. Chandramohana, J. Subramaniama, D. Dinesha, R. Rajaganesha, M. Paulpandia, H. Weic, A. T. Azizb, M. S. Alsalhid, S. Devanesand, M. Nicolettie, R. Pavelaf, and A. Canaleg (2016). Res. Vet. Sci. 106, 14.
G. Benelli, A. L. Iacono, A. Canale, and H. Mehlhorn (2016). Parasitol. Res. 115, 2131.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vijayashree, I.S., Niranjana, P., Prabhu, G. et al. Conjugation of Au Nanoparticles with Chlorambucil for Improved Anticancer Activity. J Clust Sci 28, 133–148 (2017). https://doi.org/10.1007/s10876-016-1053-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-016-1053-4