Abstract
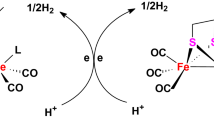
As the active site models of [FeFe]-hydrogenase, two new tertiary phosphine-substituted diiron propanedithiolate complexes [(μ-PDT)Fe2(CO)5L] (PDT = SCH2CH2CH2S, L = P(PhMe-m)3, 1; PPh2(CH2CH2CH3), 2) have been prepared through carbonyl substitution reactions of parent complex [(μ-PDT)Fe2(CO)6] (A) with P(PhMe-m)3 or PPh2(CH2CH2CH3) in the presence of the decarbonylating agent Me3NO·2H2O in MeCN at room temperature. The new complexes 1 and 2 were fully characterized by elemental analysis, FT-IR, 1H, 13C{1H}, and 31P{1H} NMR spectroscopy, as well as for 1 by X-ray crystallography. In addition, the crystal structure of 1 has indicated that the phosphorus atom of the P(PhMe-m)3 ligand resides in an apical position of the pseudo-square-pyramidal geometry of the tertiary phosphine-coordinated Fe2 atom.



Similar content being viewed by others
References
J. F. Capon, F. Gloaguen, P. Schollhammer, and J. Talarmin (2005). Coord. Chem. Rev. 249, 1664.
C. Tard and C. J. Pickett (2009). Chem. Rev. 109, 2245.
M. Fontecave and V. Artero (2011). C. R. Chimie 14, 362.
F. Wang, W. G. Wang, H. Y. Wang, G. Si, C. H. Tung, and L. Z. Wu (2012). ACS Catal. 2, 407.
S. O. Wenk, D. J. Qian, T. Wakayama, C. Nakamura, N. Zorin, M. Rögner, and J. Miyake (2002). Int. J. Hydrogen Energy 27, 1489.
S. V. Morozov, P. M. Vignais, L. Cournac, N. A. Zorin, E. E. Karyakina, A. A. Karyakin, and S. Cosnier (2002). Int. J. Hydrogen Energy 27, 1501.
J. W. Peters, W. N. Lanzilotta, B. J. Lemon, and L. C. Seefeldt (1998). Science 282, 1853.
Y. Nicolet, C. Piras, P. Legrand, E. C. Hatchikian, and J. C. Fontecilla-Camps (1999). Structure 7, 13.
A. Le Cloirec, S. P. Best, S. C. Davies, D. J. Evans, D. L. Hughes, and C. J. Pickett (1999). Chem. Commun. 22, 2285.
H. Fan and M. B. Hall (2001). J. Am. Chem. Soc. 123, 3828.
J. X. Jian, Q. Liu, Z. J. Li, F. Wang, X. B. Li, C. B. Li, B. Liu, Q. Y. Meng, B. Chen, K. Feng, C. H. Tung, and L. Z. Wu (2013). Nat. Commun. 4, 2695.
X. F. Liu (2014). J. Organomet. Chem. 750, 117.
P. A. Lindahl and J. A. Kovacs (1999). J. Clust. Sci. 1, 29.
Y. L. Li, B. Xie, L. K. Zou, X. L. Zhang, and X. Li (2012). J. Organmet. Chem. 718, 74.
X. F. Liu and H. Q. Gao (2014). J. Clust. Sci. 25, 367.
X. F. Liu and H. Q. Gao (2014). J. Clust. Sci. 25, 495.
P. H. Zhao, Y. Q. Liu, and X. H. Li (2013). Asian J. Chem. 25, 5428.
C. G. Li, Y. Zhu, X. X. Jiao, and X. Q. Fu (2014). Polyhedron 67, 416.
L. C. Song, P. H. Zhao, Z. Q. Du, M. Y. Tang, and Q. M. Hu (2010). Organometallic 29, 5751.
L. C. Song, X. J. Sun, P. H. Zhao, J. P. Li, and H. B. Song (2012). Dalton Trans. 41, 8941.
P. H. Zhao, Y. Q. Liu, and G. Z. Zhao (2013). Polyhedron 53, 144.
P. H. Zhao, Y. F. Liu, K. K. Xiong, and Y. Q. Liu (2014). J. Clust. Sci. doi:10.1007/s10876-014-0689-1.
E. J. Lyon, I. P. Georgakaki, J. H. Reibenspies, and M. Y. Darensbourg (1999). Angew. Chem. Int. Ed. 38, 3178.
CRYSTALCLEAR 1.3.6. (2005) Rigaku and Rigaku/MSC. The Woodlands, TX.
G. M. Sheldrick SHELXS97, A Program for Crystal Structure Solution (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXL97, A Program for Crystal Structure Refinement (University of Göttingen, Germany, 1997).
D. Chong, I. P. Georgakaki, R. Mejia-Rodriguez, J. Sanabria-Chinchilla, M. P. Soriaga, and M. Y. Darensbourg (2003). J. Chem. Soc. Dalton Trans. 21, 4158.
P. Li, M. Wang, C. J. He, G. H. Li, X. Y. Liu, C. N. Chen, B. Åkermark, and L. Sun (2005). Eur. J. Inorg. Chem. 2506.
Z. Wang, W. F. Jiang, J. H. Liu, W. N. Jiang, Y. Wang, B. Åkermark, and L. Sun (2008). J. Organomet. Chem. 693, 2828.
Z. B. Zhao, M. Wang, W. B. Dong, P. Li, Z. Yua, and L. Sun (2009). J. Organomet. Chem. 694, 2309.
X. F. Liu and H. Q. Gao (2013). Polyhedron 65, 1.
Y. C. Liu, C. H. Lee, G. H. Lee, and M. H. Chiang (2011). Eur. J. Inorg. Chem. 17, 1155.
Y. C. Liu, T. H. Yen, Y. J. Tseng, C. H. Hu, G. H. Lee, and M. H. Chiang (2012). Inorg. Chem. 51, 5997.
R. Mejia-Rodriguez, D. Chong, J. H. Reibenspies, M. P. Soriaga, and M. Y. Darensbourg (2004). J. Am. Chem. Soc. 126, 12004.
J. Hou, X. J. Peng, Z. Y. Zhou, S. G. Sun, X. Zhao, and S. Gao (2006). J. Organomet. Chem. 691, 4633.
C. M. Thomas, O. Rudiger, T. B. Liu, C. E. Carson, M. B. Hall, and M. Y. Darensbourg (2007). Organometallic 26, 3976.
G. Durgaprasad, R. Bolligarla, and S. K. Das (2011). J. Organomet. Chem. 696, 3097.
M. El-khateeb, M. Harb, Q. Abu-Salem, H. Görls, and W. Weigand (2013). Polyhedron 61, 1.
P. H. Zhao and Y. F. Liu (2013). Mol. Cryst. Liq. Cryst. 587, 113.
Y. F. Liu, W. J. Liang, P. H. Zhao, X. H. Li, S. N. Liu, and Y. Q. Liu (2014). Mol. Cryst. Liq. Cryst. doi:10.1080/15421406.2013.875740.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21301160) and the Natural Science Foundation for Young Scholars of Shanxi Province (No. 2012021007-4).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, PH., Liu, SN., Liu, YF. et al. Synthesis, Characterization, and Crystal Structure of Tertiary Phosphine-Substituted Diiron Propanedithiolate Complexes. J Clust Sci 25, 1331–1340 (2014). https://doi.org/10.1007/s10876-014-0711-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-014-0711-7