Abstract
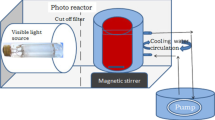
This study introduces a low temperature surfactant-free hydrothermal method to synthesize mesoporous Nb2O5 photocatalysts using NbCl5 and H2O2 as precursors that are subsequently calcinated at 300, 400 and 450 °C and are assigned as mNb2O5-300, mNb2O5-400 and mNb2O5-450, respectively. Commercial niobia sample was used as reference sample for comparison purpose. All of materials were characterized by XRD, SEM, UV–Vis DRS, FTIR, TG/DTG and BET techniques. The synthesized Nb2O5 particles especially mNb2O5-300 sample shows a high surface area (240 m2/g), a large pore volume (0.21 cm3/g) and an identifying morphology of these features. Photocatalytic decomposition of terephthalic acid was evaluated using UV–Vis spectrophotometer. The photocatalytic reactions followed pseudo-first-order kinetics with an apparent rate constant of k = 105 × 10−3 min−1 for mNb2O5-300 sample with the highest activity among all samples at natural pH (pH = 6). Meanwhile, it was observed that optimum pH of 4 resulted in fast photocatalytic reaction for mNb2O5-300 sample.











Similar content being viewed by others
References
C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli, and J. S. Beck (1992). Nature 359, (6397), 710–712.
A. Firouzi, D. Kumar, L. Bull, T. Besier, P. Sieger, Q. Huo, S. Walker, J. Zasadzinski, C. Glinka, and J. Nicol (1995). Science 267, (5201), 1138–1143.
P. T. Tanev, M. Chibwe, and T. J. Pinnavaia (1994). Nature 368, (6469), 321–323.
D. M. Antonelli and J. Y. Ying (1995). Angew. Chem. Int. Ed. 34, (18), 2014–2017.
J. Y. Ying, C. P. Mehnert, and M. S. Wong (1999). Angew. Chem. Int. Ed. 38, (1–2), 56–77.
Y. Yamauchi (2013). J. Ceram. Soc. Jpn. 121, (1417), 831–840.
Y. Yamauchi, N. Suzuki, L. Radhakrishnan, and L. Wang (2009). Chem. Rec. 9, (6), 321–339.
P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka, and G. D. Stucky (1998). Nature 396, (6707), 152–155.
M. Schmitt, S. Heusing, M. A. Aegerter, A. Pawlicka, and C. Avellaneda (1998). Sol. Energy Mater. Sol. Cells 54, (1–4), 9–17.
T. Hyodo, J. Ohoka, Y. Shimizu, and M. Egashira (2006). Sens. Actuators B Chem. 117, (2), 359–366.
H. Szymanowski, O. Zabeida, J. E. Klemberg-Sapieha, and L. Martinu (2005). J. Vac. Sci. Technol. A 23, (2), 241–247.
S.-R. Yu, X.-P. Zhang, Z.-M. He, Y.-H. Liu, and Z.-H. Liu (2004). J. Mater. Sci. Mater. Med. 15, (6), 687–691.
N. Kumagai, K. Tanno, T. Nakajima, and N. Watanabe (1983). Electrochim. Acta 28, (1), 17–22.
S. Furukawa, T. Shishido, K. Teramura, and T. Tanaka (2011). J. Phys. Chem. C 115, (39), 19320–19327.
S. Furukawa, Y. Ohno, T. Shishido, K. Teramura, and T. Tanaka (2011). ACS Catal. 1, (10), 1150–1153.
D. M. Antonelli, A. Nakahira, and J. Y. Ying (1996). Inorg. Chem. 35, (11), 3126–3136.
B. Lee, T. Yamashita, D. Lu, J. N. Kondo, and K. Domen (2002). Chem. Mater. 14, (2), 867–875.
B. Ye, M. Trudeau, and D. Antonelli (2001). Adv. Mater. 13, (1), 29–33.
Q. X. Dai, H. Y. Xiao, W. S. Li, Y. Q. Na, and X. P. Zhou (2005). Appl. Catal. A Gen. 290, (1), 25–35.
H. Kominami, K. Oki, M. Kohno, S.-I. Onoue, Y. Kera, and B. Ohtani (2001). J. Mater. Chem. 11, (2), 604–609.
D. M. Antonelli (1999). Microporous Mesoporous Mater. 33, (1–3), 209–214.
N. Suzuki, T. Athar, Y.-T. Huang, K. Shimasaki, N. Miyamoto, and Y. Yamauchi (2011). J. Ceram. Soc. Jpn. 119, (1390), 405–411.
N. Suzuki, M. Imura, Y. Nemoto, X. Jiang, and Y. Yamauchi (2011). CrystEngComm 13, (1), 40–43.
N. Suzuki, T. Kimura, and Y. Yamauchi (2010). J. Mater. Chem. 20, (25), 5294–5300.
B. Lee, D. Lu, J. N. Kondo, and K. Domen (2001). Chem. Commun. 20, 2118–2119.
B. Lee, D. Lu, J. N. Kondo, and K. Domen (2002). J. Am. Chem. Soc. 124, (38), 11256–11257.
B. N. Patil, D. B. Naik, and V. S. Shrivastava (2011). Desalination 269, (1), 276–283.
C. A. Leon y Leon, J. M. Solar, V. Calemma, and L. R. Radovic (1992). Carbon 30, (5), 797–811.
I. Nowak and M. Ziolek (1999). Chem. Rev. 99, (12), 3603–3624.
J.-M. Jehng and I. E. Wachs (1990). Catal. Today 8, (1), 37–55.
S. M. Maurer and E. I. Ko (1992). J. Catal. 135, (1), 125–134.
C. Kormann, D. W. Bahnemann, and M. R. Hoffmann (1988). J. Phys. Chem. 92, (18), 5196–5201.
Y. Sorek, R. Reisfeld, and A. M. Weiss (1995). Chem. Phys. Lett. 244, (5–6), 371–378.
L. Brus (1986). J. Phys. Chem. 90, (12), 2555–2560.
J. Lin, J. Lin, and Y. Zhu (2007). Inorg. Chem. 46, (20), 8372–8378.
M. Anpo and M. Takeuchi (2003). J. Catal. 216, (1), 505–516.
J.-H. Sun, Y.-K. Wang, R.-X. Sun, and S.-Y. Dong (2009). Mater. Chem. Phys. 115, (1), 303–308.
G. Newcombe, R. Hayes, and M. Drikas (1993). Colloids Surf. A 78, 65–71.
J. Rivera-Utrilla, I. Bautista-Toledo, and C. Moreno-Castilla (2001). J. Chem. Technol. Biotechnol. 76, (12), 1209–1215.
A. G. S. Prado and L. L. Costa (2009). J. Hazard. Mater. 169, (1), 297–301.
N. Daneshvar, S. Aber, M. S. S. Dorraji, A. R. Khataee, and M. H. Rasoulifard (2007). Sep. Purif. Technol. 58, (1), 91–98.
W. Z. Tang, Z. Zhang, H. An, M. O. Quintana, and D. F. Torres (1997). Environ. Technol. 18, (1), 1–12.
I. Peternel, N. Koprivanac, and I. Grcic (2011). Environ. Technol. 33, (1), 27–36.
I. Poulios and I. Aetopoulou (1999). Environ. Technol. 20, (5), 479–487.
J. M. Fanchiang and D. H. Tseng (2009). Environ. Technol. 30, (2), 161–172.
Y. Abdollahi, A. H. Abdullah, U. I. Gaya, Z. Zainal, and N. A. Yusof (2011). Environ. Technol. 33, (10), 1183–1189.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Houshang, F., Fatemeh, H., Rahmatollah, R. et al. Surfactant-Free Hydrothermal Synthesis of Mesoporous Niobia Samples and Their Photoinduced Decomposition of Terephthalic Acid (TPA). J Clust Sci 25, 651–666 (2014). https://doi.org/10.1007/s10876-013-0661-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0661-5