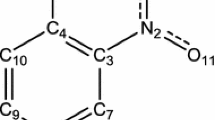
Abstract
In this work, the intermolecular dihydrogen and hydrogen bonding interactions in electronically excited states of a 2-pyridone (2PY)–borane–trimethylamine (BTMA) cluster have been theoretically studied using time-dependent density functional theory method. Our computational results show that the S1 state of 2PY–BTMA cluster is a locally excited state, in which only 2PY moiety is electronically excited. The theoretical infrared (IR) spectra of the 2PY–BTMA cluster demonstrate that the N–H stretching vibrational mode is slightly blue-shifted upon the electronic excitation. Moreover, the computed IR spectrum of the 2PY–BTMA cluster exhibits no carbonyl character due to the extension of the C=O bond length in the S1 state. However, the N–H bond is shortened slightly upon photoexcitation. At the same time, the H···H and H···O distances are obviously lengthened in the S1 sate by comparison with those in ground state. In addition, the electron density of the carbonyl oxygen is diminished due to the electronic excitation. Consequently, the proton acceptor ability of carbonyl oxygen is decreased in the electronic excited state. As a result, it is demonstrated that the intermolecular dihydrogen and hydrogen bonds are significantly weakened in the electronically excited state.





Similar content being viewed by others
References
K. L. Han and G. J. Zhao, Hydrogen Bonding and Transfer in the Excited State, (Wiley, Chichester, 2011), Vol. I & II (ISBN: 978-0-470-66677-7).
H. Zhang, S. F. Wang, Q. Sun, and S. C. Smith (2009). Phys. Chem. Chem. Phys. 11, 8422.
L. Zhou, G. Zhao, J. Liu, K. Han, Y. Wu, X. Peng, and M. Sun (2007). J. Photochem. Photobiol. A 187, 305–310.
G. J. Zhao and K. L. Han (2007). J. Phys. Chem. A 111, 9218.
G. J. Zhao and K. L. Han (2007). J. Phys. Chem. A 111, 2469.
Y. F. Liu, Y. G. Yang, K. Jiang, D. H. Shi, and J. F. Sun (2011). Phys. Chem. Chem. Phys. 13, 15299.
J. Chen, G. Zhao, X. Sun, S. Yang, M. Zhang, K. Han, and P. J. Stang (2012). J. Phys. Chem. A 116, 9911–9918.
Y. F. Liu, J. X. Ding, R. Q. Liu, D. H. Shi, and J. F. Sun (2009). J. Photochem. Photobiol. A 201, 203.
D. D. Wang, C. Hao, S. Wang, H. Dong, and J. S. Qiu (2012). J. Mol. Model. 18, 937.
G. J. Zhao, J. Y. Liu, L. C. Zhou, and K. L. Han (2007). J. Phys. Chem. B 111, 8940.
G. J. Zhao and K. L. Han (2008). Biophys. J. 94, 38.
G. J. Zhao and K. L. Han (2008). J. Comput. Chem. 29, 2010.
G. J. Zhao and K. L. Han (2008). ChemPhysChem 9, 1842.
G. J. Zhao and K. L. Han (2009). J. Phys. Chem. A 113, 14329.
G. J. Zhao, K. L. Han, and P. J. Stang (2009). J. Chem. Theory Comput. 5, 1955.
G. J. Zhao and K. L. Han (2012). Acc. Chem. Res. 45, 404.
Y. F. Liu, J. X. Ding, D. H. Shi, and J. F. Sun (2008). J. Phys. Chem. A 112, 6244.
Y. F. Liu, J. X. Ding, R. Q. Liu, D. H. Shi, and J. F. Sun (2009). J. Comput. Chem. 30, 2723.
G. J. Zhao, F. Yu, M. Zhang, B. H. Northrop, H. Yang, K. L. Han, and P. J. Stang (2011). J. Phys. Chem. A 115, 6390.
F. Yu, P. Li, G. Li, G. Zhao, T. Chu, and K. Han (2011). J. Am. Chem. Soc. 133, 11030.
G. J. Zhao, B. H. Northrop, K. L. Han, and P. J. Stang (2010). J. Phys. Chem. A 114, 9007.
L. C. Zhou, J. Y. Liu, G. J. Zhao, Y. Shi, X. J. Peng, and K. L. Han (2007). Chem. Phys. 333, 179.
S. Chai, G.-J. Zhao, P. Song, S.-Q. Yang, J.-Y. Liu, and K.-L. Han (2009). Phys. Chem. Chem. Phys. 11, 4385.
Y.-H. Liu, G.-J. Zhao, G.-Y. Li, and K.-L. Han (2010). J. Photochem. Photobiol. A 209, 181.
G. Zhao, R. Chen, M. Sun, G. Li, J. Liu, Y. Gao, K. Han, X. Yang, and L. Sun (2008). Chem. Eur. J. 14, 6935.
R. H. Crabtree, O. Eisenstein, G. Sini, and E. Peris (1998). J. Org. Chem. 567, 7.
G. Zhao and C. Cheng (2012). Amino Acids 43, 557–565.
K. L. Han and G. Z. He (2007). J. Photochem. Photobiol. C 8, 55–66.
J. P. Campbell, J. W. Hwang, V. G. Young, R. B. Von Dreele, C. J. Cramer, and W. L. Gladfelter (1998). J. Am. Chem. Soc. 120, 521.
C. Cheng and G. Zhao (2012). Nanoscale 4, 2301–2305.
C. F. Matta, J. Hernández-Trujillo, T.-H. Tang, and R. F. W. Bader (2003). Chem. Eur. J. 9, 1940.
G. J. Zhao, K. Han, Y. Lei, and Y. Dou (2007). J. Chem. Phys. 127, 094307.
R. H. Crabtree, P. M. Siegbahn, O. Eisenstein, A. Rheingold, and T. F. Koetzle (1996). Acc. Chem. Res. 29, 348.
G. Zhao, B. Northrop, P. Stang, and K. Han (2010). J. Phys. Chem. A 114, 3418–3422.
T. B. Richardson, S. de Gala, R. H. Crabtree, and P. E. M. Siegbhan (1995). J. Am. Chem. Soc. 117, 12875.
G. Zhao and K. Han (2010). Phys. Chem. Chem. Phys. 12, 8914–8918.
L. M. Epstein, E. S. Shubina, E. V. Bakhmutova, L. N. Saitkulova, V. I. Bakhmutov, A. L. Chistyakov, and I. V. Stankevich (1998). Inorg. Chem. 37, 3013.
G. Zhao and K. Han (2009). J. Phys. Chem. A 113, 4788–4794.
S. A. Kulkarni and A. K. Srivastava (1999). J. Phys. Chem. A 103, 2836.
M. Zhang and G. Zhao (2012). ChemSusChem 5, 879–887.
S. C. Gatling and J. E. Jackson (1999). J. Am. Chem. Soc. 121, 8655.
G. N. Patwari, T. Ebata, and N. J. Mikami (2000). J. Chem. Phys. 113, 9885.
G. N. Patwari, T. Ebata, and N. J. Mikami (2001). J. Chem. Phys. 114, 8877.
G. N. Patwari, T. Ebata, and N. J. Mikami (2002). Chem. Phys. 116, 6056.
G. N. Patwari, T. Ebata, and N. Mikami (2002). Chem. Phys. 283, 193.
G. N. Patwari (2005). J. Phys. Chem. A 109, 2035.
G. N. Patwari, T. Ebata, and N. Mikami (2001). J. Phys. Chem. A 105, 8642.
G. N. Patwari, A. Fujii, and N. Mikami (2006). J. Chem. Phys. 124, 241103.
G. N. Patwari, A. Fujii, and N. Mikami (2001). J. Phys. Chem. A 105, 10753.
S. L. Gao, W. Wu, and Y. R. Mo (2009). J. Phys. Chem. A 113, 8108.
G.-J. Zhao and K.-L. Han (2007). J. Chem. Phys. 127, 024306.
N.-N. Wei, P. Li, C. Hao, R. Wang, Z.-L. Xiu, J.-W. Chen, and P. Song (2010). J. Photochem. Photobiol. A 210, 77.
N.-N. Wei, C. Hao, Z.-L. Xiu, J.-W. Chen, and J.-S. Qiu (2010). Phys. Chem. Chem. Phys. 12, 9445.
N.-N. Wei, C. Hao, Z.-L. Xiu, J.-W. Chen, and J.-S. Qiu (2010). J. Comput. Chem. 31, 2853–2858.
N.-N. Wei, C. Hao, J.-J. Tan, G. Zhao, R. Li, Z.-L. Xiu, and J.-S. Qiu (2011). J. Mol. Model. 17, 1891.
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
A. Schäfer, C. Huber, and R. Ahlrichs (1994). J. Chem. Phys. 100, 5829.
N. A. Besley and J. D. Hirst (1999). J. Am. Chem. Soc. 121, 8559.
N. A. Besley and J. D. Hirst (1998). J. Phys. Chem. A 102, 10791.
F. Furchea and R. Ahlrichs (2002). J. Chem. Phys. 117, 7433.
P. Deglmann, F. Furche, and R. Ahlrichs (2002). Chem. Phys. Lett. 362, 511.
P. Deglmann and F. Furche (2002). J. Chem. Phys. 117, 9535.
P. Weis, P. R. Kemper, and M. T. Bowers (1997). J. Phys. Chem. A 101, 2809.
P. Weber and J. R. Reimers (1999). J. Phys. Chem. A 103, 9830.
J. F. Beck and Y. R. Mo (2006). J. Comput. Chem. 28, 455.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21036006) and the Key Laboratory of Industrial Ecology and Environmental Engineering, China Ministry of Education.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wei, N., Hamza, A., Hao, C. et al. Time-Dependent Density Functional Theory Study on Hydrogen and Dihydrogen Bonding in Electronically Excited State of 2-Pyridone–Borane–Trimethylamine Cluster. J Clust Sci 24, 459–470 (2013). https://doi.org/10.1007/s10876-013-0572-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0572-5