Abstract
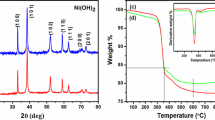
α-Ni(OH)2 flower-like nanostructures were successfully synthesized through one-step hydrothermal method with nickel acetate tetrahydrate, ethylene-1,2-diamine (en), hexamethylenetetramine (HMT) and cetyltrimethylammonium bromide (CTAB) as morphology-directing agents. Optimum conditions to obtain high yield and pure phase α-Ni(OH)2 were identified by varying experimental parameters such as: en, HMT and CTAB concentration and reaction temperature. The products were characterized by X-ray diffraction, scanning electron microscopy, Fourier transform infrared and thermogravimetric analysis. These results indicated that the α-nickel hydroxide contains water molecules and anions. The hierarchical NiO nanostructures were obtained by the as-synthesized α-Ni(OH)2 nanostructures annealed at 300 °C for 4 h.











Similar content being viewed by others
References
C. Xu, L. Wang, D. Zou, and T. Ying (2008). Mater. Lett. 62, 3181.
Y. Shao, J. Sun, and L. Gao (2009). J. Phys. Chem. C 113, 6566.
K. Xu and W. Ding (2008). Mater. Lett. 62, 4437.
C. Coudun and J. F. Hochepied (2005). J. Phys. Chem. B 109, 6069.
X. Wang, H. Luo, P. V. Parkhutik, A. C. Millan, and E. Matveeva (2003). J. Power Sources 115, 153.
J. J. Braconniera, C. Delmas, and P. Hagenmuller (1982). Mater. Res. Bull. 17, 993.
B. Liu, X. Y. Wang, H. T. Yuan, Y. S. Zhang, D. Y. Song, and Z. X. Zhou (1999). J. Appl. Electrochem. 29, 855.
I. Zhitomirsky (2004). J. Appl. Electrochem. 34, 235.
W. Y. Li, S. Y. Zhang, and J. Chen (2005). J. Phys. Chem. B 109, 14025.
R. Acharya, T. Subbaiah, S. Anand, and R. P. Das (2003). Mater. Chem. Phys. 81, 45.
A. Delahaye-Vidal and M. Figiarz (1987). J. Appl. Electrochem. 17, 589.
M. Dixit, G. N. Subbanna, and P. V. Kamath (1996). J. Mater. Chem. 6, 1429.
Y. L. Zhao, J. M. Wang, H. Chen, T. Pan, J. Q. Zhang, and C. N. Cao (2004). Int. J. Hydrogen Energy 29, 889.
F. Portemer, A. Delahaye-Vidal, and M. Figiarz (1992). J. Electrochem. Soc. 139, 671.
M. Akine, N. Jongen, J. Lemaitre, and H. Hofmann (1998). J. Eur. Ceram. Soc. 18, 1559.
M. Yoshio, Y. Todorov, K. Yamato, H. Noguchi, J. Itoh, M. Okada, and T. Mouri (1998). J. Power Sources 74, 46.
B. Sheela, H. Gomathi, and G. P. Rao (1995). J. Electroanal. Chem. 394, 267.
C. B. Alcock, B. Z. Li, J. W. Fergus, and L. Wang (1992). Solid State Ionics 53, 39.
A. C. Felici, F. Lama, M. Piacentini, T. Papa, D. Debowska, and A. Kisiel (1996). J. Appl. Phys. 80, 6925.
D. Adler and J. Feinleib (1970). Phys. Rev. B 2, 3112.
X. Wang, J. M. Song, L. S. Gao, J. Y. Jin, H. G. Zheng, and Z. D. Zhang (2005). Nanotechnology 16, 37.
F. Mohandes and M. Salavati-Niasari (2013). Ultrason. Sonochem. 20, 354.
F. Soofivand, F. Mohandes, and M. Salavati-Niasari (2012). Micro Nano Lett. 7, 283.
M. Jafari, M. Salavati-Niasari, and F. Mohandes (2012). J. Inorg. Organomet. Polym.. doi:10.1007/s10904-012-9784-7.
S. M. Hosseinpour-Mashkani, F. Mohandes, M. Salavati-Niasari, and K. Venkateswara-Rao (2012). Mater. Res. Bull. 47, 3148.
F. Davar, F. Mohandes, and M. Salavati-Niasari (2010). Polyhedron 29, 3132.
N. Bouropoulos, G. C. Psarras, N. Moustakas, A. Chrissanthopoulos, and S. Baskoutas (2008). Phys. Status Solidi A 205, 2033.
F. Mohandes, F. Davar, and M. Salavati-Niasari (2010). J. Magn. Magn. Mater. 322, 872.
M. Salavati-Niasari, F. Mohandes, F. Davar, and K. Saberyan (2009). Appl. Sur. Sci. 256, 1476.
S. Baskoutas, P. Giabouranis, S. N. Yannopoulos, V. Dracopoulos, L. Toth, A. Chrissanthopoulos, and N. Bouropoulos (2007). Thin Solid Films 515, 8461.
D. Yang, R. Wang, M. He, J. Zhang, and Z. Liu (2005). J. Phys. Chem. B 109, 7654.
M. Jayalakshmi, N. Venugopal, B. R. Reddy, and M. M. Rao (2005). J. Power Source 150, 272.
Z. H. Liang, Y. J. Zhu, and X. L. Hu (2004). J. Phys. Chem. B 108, 3488.
W. K. Hu, X. P. Gao, D. Noreus, T. Burchardt, and N. Nakstad (2006). J. Power Source 160, 704.
H. Zhang, H. Liu, X. Cao, S. Li, and C. Sun (2003). Mater. Chem. Phys. 79, 37.
E. Esmaeili, M. Salavati-Niasari, F. Mohandes, F. Davar, and H. Seyghalkar (2011). Chem. Eng. J. 170, 278.
M. Salavati-Niasari, F. Davar, and Z. Fereshteh (2010). J. Alloys Compd. 494, 410.
M. Salavati-Niasari, N. Mir, and F. Davar (2010). J. Alloys Compd. 493, 163.
N. V. Kosova, E. T. Devyatkina, and V. V. Kaichev (2007). J. Power Source 174, 735.
L. Xu, Y.-S. Ding, C.-H. Chen, L. Zhao, C. Rimkus, R. Joesten, and S. L. Suib (2008). Chem. Mater. 20, 308.
L.-X. Yang, Y. J. Zhu, H. Tong, Z. H. Liang, L. Li, and L. Zhang (2007). J. Solid State Chem. 180, 2095.
Acknowledgments
Authors are grateful to the council of Iran National Science Foundation and University of Kashan for supporting this work by Grant No (159271/51).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salavati-Niasari, M., Seyghalkar, H., Amiri, O. et al. Simple Hydrothermal Synthesis of Nickel Hydroxide Flower-Like Nanostructures. J Clust Sci 24, 365–376 (2013). https://doi.org/10.1007/s10876-013-0558-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0558-3