Abstract
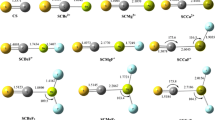
Bond distances, vibrational frequencies, dissociation energies, electron affinities, ionization potentials and dipole moments of the title molecules in neutral and charged ions were studied by use of density functional method. Ground states for each molecule were assigned. The calculated bond distance decreases with the increasing of atomic number of 4d metals, reaches minimum at RhS, then increases. For cationic molecules, the calculated bond distance decreases to the minimum at MoS+, then increases. The calculated vibrational frequency decreases from YS(YS+) to PdS(PdS+) for both neutral and cationic molecules. The bond ionic character decreases from YS(YS+) to PdS(PdS+) for neutral and cationic molecules. The bonding patterns are discussed and compared with the available studies.
Similar content being viewed by others
References
E. I. Stiefel and K. Matsumoto (eds.), Transition Metal Sulfur Chemistry; ACS Symposium Series 653 (American Chemistry Society, Washington, DC, 1996).
M. Hua, C. I. Garcia, and A. DeArdo (1997). J. Metall. Mater. Trans. A 28A, 1769.
T. C. Steimle and W. Virgo (2003). J. Mol. Spectrosc. 221, 57.
P. Cheng, G. K. Koyanagi, and D. K. Bohme (2006). J. Phys. Chem. A 110, 2718.
I. Kretzschmar, D. Schroder, H. Schwarz, and P. B. Armentrout (2006). Int. J. Mass Spectrom. 249–250, 263.
S. A. Beaton and M. C. L. Gerry (1999). J. Chem. Phys. 110, 10715.
R. R. Bousquet, K. C. Namiki, and T. C. Steimle (2000). J. Chem. Phys. 113, 1566.
R. R. Reddy, Y. N. Ahammed, B. S. Devi, K. R. Gopal, P. A. Azeem, and T. V. R. Rao (2002). Astrophys. Space Sci. 281, 729.
B. Liang and L. Andrews (2002). J. Phys. Chem. A 106, 6295.
B. Liang and L. Andrews (2002). J. Phys. Chem. A 106, 3738.
O. Launila (2005). J. Mol. Spectrosc. 229, 31.
R. Oviedo-Roa, J. M. Martinez-Magadan, and F. Illas (2006). J. Phys. Chem. B 110, 7951.
B. Liang and L. Andrews (2002). J. Phys. Chem. A 106, 6945.
R. Li, W. J. Balfour, W. S. Hopkins, and A. G. Adam (2005). J. Mol. Spectrosc. 234, 211.
P. B. Armentrout (2003). Int. J. Mass Spectrom. 227, 289.
K. A. Peterson, B. C. Shepler, and J. M. Singleton (2007). Mol. Phys. 105, 445.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, J. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision B.02 (Gaussian, Inc., Pittsburgh PA, 2003).
A. D. Becke (1993). J. Chem. Phys. 98, 5648.
C. Lee, W. Yang, and R. G. Parr (1988). Phys. Rev. B 37, 785.
P. J. Hay and W. R. Wadt (1985). J. Chem. Phys. 82, 299.
A. M. James and B. Simard (1993). J. Chem. Phys. 98, 4422.
S. R. Langhoff and C. W. Bauschlicher Jr. (1988). J. Chem. Phys. 89, 2160.
J. Jonsson, B. Lindgren, and A. G. Taklif (1991). Astron. Astrophys. 246, L67.
Acknowledgment
JPW and XBS thank the Ph.D. Foundation of Qingdao Agricultural University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, X., Wang, J. & Wu, Z. Chemical Bonding and Electronic Structure of 4d-Metal Monosulfides. J Clust Sci 20, 525–534 (2009). https://doi.org/10.1007/s10876-009-0252-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-009-0252-7