Abstract
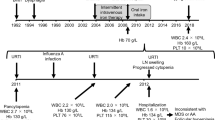
Cytotoxic T-lymphocyte-antigen 4 (CTLA-4) is an essential negative regulator expressed on regulatory T cells (Tregs) and activated T cells. Germline heterozygous mutations in CTLA4 lead to haploinsufficiency of CTLA-4, resulting in the development of an autosomal dominant immune dysregulation syndrome with incomplete penetrance. We report here a Japanese patient with this disorder who has a novel heterozygous single nucleotide insertion, 76_77insT (p. L28SfsX40), in the CTLA4 gene. Peripheral blood mononuclear cells from the patient showed decreased frequency of CTLA-4high cells in CD4+FOXP3+ cells following CD3/CD28 stimulation. The patient experienced hypogammaglobulinemia, recurrent pneumonia, esophageal candidiasis, cytomegalovirus-positive chronic gastritis, chronic and severe diarrhea, and type 1 diabetes mellitus. Moreover, the patient developed multifocal gastric cancer, histologically poorly and well-differentiated adenocarcinomas, associated with chronic atrophic gastritis and intestinal metaplasia. Previously, 23 symptomatic cases with heterozygous CTLA4 mutations have been reported. Including the case presented here, 3 of the 24 cases (12.5 %) developed gastric cancer. Notably, 2 of 3 patients presented similarly multifocal adenocarcinomas associated with atrophic gastritis and intestinal metaplasia. Predisposition to gastric cancer has been also reported in CVID patients. These clinical observations suggest that gastric cancer is a disease commonly associated with autosomal dominant immune dysregulation syndrome due to CTLA4 mutation.


Similar content being viewed by others
References
Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–7.
Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–6.
Bakacs T, Mehrishi JN. Anti-CTLA-4 therapy may have mechanisms similar to those occurring in inherited human CTLA4 haploinsufficiency. Immunobiology. 2015;220(5):624–5.
Kamae C, Nakagawa N, Sato H, Honma K, Mitsuiki N, Ohara O, et al. Common variable immunodeficiency classification by quantifying T-cell receptor and immunoglobulin kappa-deleting recombination excision circles. J Allergy Clin Immunol. 2013;131(5):1437–40 e5.
Compare D, Rocco A. Nardone G risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14(4):302–8.
Zeissig S, Petersen BS, Tomczak M, Melum E, Huc-Claustre E, Dougan SK, et al. Early-onset Crohn's disease and autoimmunity associated with a variant in CTLA-4. Gut. 2014;64(12):1889–97.
Dhalla F, da Silva SP, Lucas M, Travis S. Chapel H review of gastric cancer risk factors in patients with common variable immunodeficiency disorders, resulting in a proposal for a surveillance programme. Clin Exp Immunol. 2011;165(1):1–7.
Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130(3):495–500.
Acknowledgments
Sequence analysis was supported by the Analysis Center of Life Science, Natural Science Center for Basic Research and Development, Hiroshima University (Hiroshima, Japan). This study was supported in part by Grants in Aid for Scientific Research from the Japan Society for the Promotion of Science [25293231 to M.K.], [26670501 to M.K.], [25713039 to S.O.], and [25670477 to S.O.]. This study was also supported in part by Research on Measures for Intractable Diseases funding from the Japanese Ministry of Health, Labour and Welfare [201324051A to M.K.] and [201324062A to M.K.].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayakawa, S., Okada, S., Tsumura, M. et al. A Patient with CTLA-4 Haploinsufficiency Presenting Gastric Cancer. J Clin Immunol 36, 28–32 (2016). https://doi.org/10.1007/s10875-015-0221-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0221-x