Abstract
Purpose
Mycobacterium leprae exploits complement activation and opsonophagocytosis to infect phagocytes. M-ficolin is encoded by the FCN1 gene and initiates the lectin pathway on monocyte surfaces. We investigated FCN1 promoter polymorphisms that could be responsible for the high interindividual variability of M-ficolin levels and for modulating leprosy susceptibility.
Methods
We genotyped rs2989727 (−1981 G > A), rs28909068 (−791 G > A), rs10120023 (−542 G > A), rs17039495 (−399 G > A), rs28909976 (−271IndelT), rs10117466 (−144C > A) and rs10858293 (+33 T > G) in 400 controls and 315 leprosy patients from Southern Brazil, and in 296 Danish healthy individuals with known M-ficolin levels.
Results
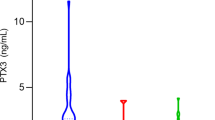
Ten haplotypes were identified with sequence-specific PCR and/or haplotype-specific sequencing. We found evidence for a protective codominant additive effect of FCN1*−542A–144C with leprosy in Euro-Brazilians (P = 0.003, PBf = 0.021, OR = 0.243 [CI95% = 0.083–0.71]), which was independent of age, ethnic group and gender effects (P = 0.029). There was a trend for a positive association of the −399A variant in Afro-Brazilians (P = 0.022, PBf = 0.154, OR = 4.151 [CI95% = 1.115–15.454], as well as for a negative association of the FCN1*3A haplotype with lepromatous leprosy, compared with less severe forms of the disease (P = 0.016, PBf = 0.112, OR = 0.324 [CI95% = 0.123–0.858]). Danish individuals with this haplotype presented M-ficolin levels higher than the population average of circa 1,000 ng/ml, and −542A–144C, which is able to modify the recognition of transcription factors in silico, occurred in individuals with levels under the 25 percentil (P = 0.031).
Conclusions
Our data provide the first evidence that FCN1 polymorphisms are associated with leprosy. M-ficolin may represent a novel key to understand the immunopathogenesis of M. leprae infection.





Similar content being viewed by others
References
Alter A, Alcais A, Abel L, Schurr E. Leprosy as a genetic model for susceptibility to common infectious diseases. Hum Genet. 2008;123:227–35.
Teh C, Le Y, Lee SH, Lu J. M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-D-glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli. Immunology. 2000;101:225–32.
Frederiksen PD, Thiel S, Larsen CB, Jensenius JC. M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol. 2005;62:462–73.
Liu Y, Endo Y, Iwaki D, Nakata M, Matsushita M, Wada I, et al. Human M-ficolin is a secretory protein that activates the lectin complement pathway. J Immunol. 2005;175:3150–6.
Rorvig S, Honore C, Larsson LI, Ohlsson S, Pedersen CC, Jacobsen LC, et al. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J Leukoc Biol. 2009;86:1439–49.
Honore C, Rorvig S, Munthe-Fog L, Hummelshoj T, Madsen HO, Borregaard N, et al. The innate pattern recognition molecule Ficolin-1 is secreted by monocytes/macrophages and is circulating in human plasma. Mol Immunol. 2008;45:2782–9.
Xu P, Crawford M, Way M, Godovac-Zimmermann J, Segal AW, Radulovic M. Subproteome analysis of the neutrophil cytoskeleton. Proteomics. 2009;9:2037–49.
Wittenborn T, Thiel S, Jensen LT, Nielsen HJ, Jensen L, Jensenius JC. Characteristics and biological variations of M-ficolin, a pattern recognition molecule, in plasma. J Innate Immun. 2009;2:167–80.
Runza VL, Hehlgans T, Echtenacher B, Zahringer U, Schwaeble WJ, Mannel DN. Localization of the mouse defense lectin ficolin B in lysosomes of activated macrophages. J Endotoxin Res. 2006;12:120–6.
Garlatti V, Martin L, Gout E, Reiser JB, Fujita T, Arlaud GJ, et al. Structural basis for innate immune sensing by M-ficolin and its control by a pH-dependent conformational switch. J Biol Chem. 2007;282:35814–20.
Tanio M, Kondo S, Sugio S, Kohno T. Trivalent recognition unit of innate immunity system: crystal structure of trimeric human M-ficolin fibrinogen-like domain. J Biol Chem. 2007;282:3889–95.
Lu J, Tay PN, Kon OL, Reid KB. Human ficolin: cDNA cloning, demonstration of peripheral blood leucocytes as the major site of synthesis and assignment of the gene to chromosome 9. Biochem J. 1996;313(Pt 2):473–8.
Hummelshoj T, Munthe-Fog L, Madsen HO, Garred P. Functional SNPs in the human ficolin (FCN) genes reveal distinct geographical patterns. Mol Immunol. 2008;45:2508–20.
Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73.
Boldt AB, Petzl-Erler ML. A new strategy for mannose-binding lectin gene haplotyping. Hum Mutat. 2002;19:296–306.
Boldt AB, Messias-Reason IJ, Lell B, Issifou S, Pedroso ML, Kremsner PG, et al. Haplotype specific-sequencing reveals MBL2 association with asymptomatic Plasmodium falciparum infection. Malar J. 2009;8:97.
Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–72.
Raymond M, Rousset F. An exact test for population differentiation. Evolution. 1995;49:1280–3.
de Messias-Reason IJ, Boldt AB, Moraes Braga AC, Von Rosen Seeling SE, Dornelles L, Pereira-Ferrari L, et al. The association between mannan-binding lectin gene polymorphism and clinical leprosy: new insight into an old paradigm. J Infect Dis. 2007;196:1379–85.
de Messias-Reason I, Kremsner PG, Kun JF. Functional haplotypes that produce normal ficolin-2 levels protect against clinical leprosy. J Infect Dis. 2009;199:801–4.
Lin DY, Zeng D, Millikan R. Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol. 2005;29:299–312.
Boldt AB, Messias-Reason IJ, Meyer D, Schrago CG, Lang F, Lell B, et al. Phylogenetic nomenclature and evolution of mannose-binding lectin (MBL2) haplotypes. BMC Genet. 2010;11:38.
Boldt AB, Culpi L, Tsuneto LT, de Souza IR, Kun JF, Petzl-Erler ML. Diversity of the MBL2 gene in various Brazilian populations and the case of selection at the mannose-binding lectin locus. Hum Immunol. 2006;67:722–34.
Boldt AB, Grisbach C, Steffensen R, Thiel S, Kun JF, Jensenius JC, et al. Multiplex sequence-specific polymerase chain reaction reveals new MASP2 haplotypes associated with MASP-2 and MAp19 serum levels. Hum Immunol. 2011;72:753–60.
Huret JL, Senon S, Bernheim A, Dessen P. An atlas on genes and chromosomes in oncology and haematology. Cell Mol Biol. 2004;50:805–7.
Carroll MV, Lack N, Sim E, Krarup A, Sim RB. Multiple routes of complement activation by Mycobacterium bovis BCG. Mol Immunol. 2009;46:3367–78.
Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis. 2003;83:91–7.
Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections - another case of heterosis. Eur J Immunogenet. 1994;21:125–31.
Dornelles LN, Pereira-Ferrari L, Messias-Reason I. Mannan-binding lectin plasma levels in leprosy: deficiency confers protection against the lepromatous but not the tuberculoid forms. Clin Exp Immunol. 2006;145:463–8.
Gomes GI, Nahn EP, Santos RKRG, Da Silva WD, Kipnis TL. The functional state of the complement system in leprosy. AmJTrop Med Hyg. 2008;78:605–10.
de Messias I, Santamaria J, Brenden M, Reis A, Mauff G. Association of C4B deficiency (C4B*Q0) with erythema nodosum in leprosy. Clin Exp Immunol. 1993;92:284–7.
Frankenberger M, Schwaeble W, Ziegler-Heitbrock L. Expression of M-Ficolin in human monocytes and macrophages. Mol Immunol. 2008;45:1424–30.
Bochud PY, Hawn TR, Aderem A. Cutting edge: a toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170:3451–4.
Bochud PY, Hawn TR, Siddiqui MR, Saunderson P, Britton S, Abraham I, et al. Toll-like receptor 2 (TLR2) polymorphisms are associated with reversal reaction in leprosy. J Infect Dis. 2008;197:253–61.
Bochud PY, Sinsimer D, Aderem A, Siddiqui MR, Saunderson P, Britton S, et al. Polymorphisms in Toll-like receptor 4 (TLR4) are associated with protection against leprosy. Eur J Clin Microbiol Infect Dis. 2009;28:1055–65.
Garred P, Honore C, Ma YJ, Rorvig S, Cowland J, Borregaard N, et al. The genetics of ficolins. J Innate Immun. 2010;2:3–16.
Vander Cruyssen B, Nuytinck L, Boullart L, Elewaut D, Waegeman W, Van TM, et al. Polymorphisms in the ficolin 1 gene (FCN1) are associated with susceptibility to the development of rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1792–5.
Schlapbach LJ, Thiel S, Aebi C, Hirt A, Leibundgut K, Jensenius JC, et al. M-ficolin in children with cancer. Immunobiology. 2011;216:633–8.
Bodmer W, Tomlinson I. Rare genetic variants and the risk of cancer. Curr Opin Genet Dev. 2010;20:262–7.
Schlapbach LJ, Kessler U, Thiel S, Hansen AG, Nelle M, Ammann RA, et al. M-ficolin in the neonatal period: associations with need for mechanical ventilation and mortality in premature infants with necrotising enterocolitis. Mol Immunol. 2009;46:2597–603.
Steffensen R, Thiel S, Varming K, Jersild C, Jensenius JC. Detection of structural gene mutations and promoter polymorphisms in the mannan-binding lectin (MBL) gene by polymerase chain reaction with sequence-specific primers. J Immunol Methods. 2000;241:33–42.
Boldt AB, Luty A, Grobusch MP, Dietz K, Dzeing A, Kombila M, et al. Association of a new mannose-binding lectin variant with severe malaria in Gabonese children. Genes Immun. 2006;7:393–400.
Akaike H. Prediction and entropy. In: Atkinson AC, Fienberg SE, editors. A celebration of statistics. New York: Springer; 1985. p. 1–24.
Nebert DW. Proposal for an allele nomenclature system based on the evolutionary divergence of haplotypes. Hum Mutat. 2002;20:463–72.
Acknowledgments
The subjects of this investigation were informed about the aims of the study and their consent to participate is gratefully acknowledged. We are also thankful to the medical staff of the Hospital de Clínicas of the Federal University of Paraná and of the Sanitary and Dermatologic Hospital of Paraná for patient recruitment, to the staff of the Laboratório de Imunopatologia Molecular in Curitiba and of the Department of Clinical Immunology in Aalborg for assistance in the DNA extraction, to Rubia Zem, Andressa Chequin, Bianca Oliveira and Caroline Grisbach for valuable help in the FCN1 genotyping. We are grateful to Andrea Weierich and Viola Galinat from Tübingen for DNA sequencing, as well as for the excellent technical assistance from Annette Hansen, Louise Jakobsen and Lisbeth Jensen in Aarhus. This work was supported by a PRODOC grant of CAPES (Coordenação de Aperfeiçoamento de Pessoal Superior) and by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) grants for ABW Boldt and IJT Messias-Reason, as well as by a grant of the Bundesministerium für Bildung und Forschung (BMBF) for JFJ Kun and of the Danish Research Council and Novo Nordic Foundation for S Thiel and JC Jensenius.
Conflict of interest
Dr. Thiel and Dr. Jensenius have financial interest in NatImmune A/S, a biotech company exploring the possibilities of therapy with proteins of the innate immune system. Dr. Boldt, Ms. Neves Sanchez, Dr. Steffensen, Dr. Mira, Dr. Stahlke and Dr. Messias-Reason declare no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
In memorian (28.04.2011). We miss Prof. Jürgen F. J. Kun as a wonderful friend and excellent scientist.
Rights and permissions
About this article
Cite this article
Boldt, A.B.W., Sanchez, M.I.N., Stahlke, E.R.S. et al. Susceptibility to Leprosy is Associated with M-ficolin Polymorphisms. J Clin Immunol 33, 210–219 (2013). https://doi.org/10.1007/s10875-012-9770-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9770-4