Abstract
Objectives
Objectives The objectives of this study are to (1) evaluate the ability of the immune system to synthesize specific antibodies that catalyze the degradation of amyloid β peptide (Aβ) and to (2) evaluate the prospect of developing a catalytic IVIG (CIVIG) formulation for therapy of Alzheimer’s disease (AD).
Conclusions
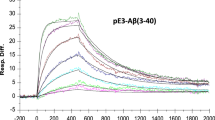
Polyclonal autoantibodies from humans without dementia hydrolyzed Aβ specifically. The catalytic activity improved as a function of age. Patients with AD produced catalytic antibodies at increased levels. IgM-class antibodies expressed the activity at levels superior to IgGs. Production of catalytic autoantibodies appears to be an innate immunity function with adaptive improvements occurring upon Aβ overexpression, which suggests a beneficial function of the catalytic activity. The catalytic autoantibodies impeded Aβ aggregation, dissolved preformed Aβ aggregates, and inhibited Aβ cytotoxicity in tissue culture. Recombinant catalytic antibodies from a human library have been identified, validating the phenomenon of antibody-catalyzed Aβ cleavage. As a single catalyst molecule inactivates multiple Aβ molecules, catalytic antibodies may clear Aβ efficiently. IVIG did not cleave Aβ, indicating the importance of purification procedures that maintain catalytic site integrity. Traditional Aβ-binding antibodies form immune complexes that can induce inflammatory reaction and vascular dysfunction. Catalysts do not form stable immune complexes, minimizing these risks. Criteria appropriate for developing a CIVIG formulation with potential therapeutic utility are discussed, including isolation of the Aβ-specific catalytic subsets present in IgM and IgG from human blood.



Similar content being viewed by others
References
Vani J, Elluru S, Negi VS, Lacroix-Desmazes S, Kazatchkine MD, Bayary J, et al. Role of natural antibodies in immune homeostasis: IVIg perspective. Autoimmun Rev. 2008;7:440–4.
Paul S, Volle DJ, Beach CM, Johnson DR, Powell MJ, Massey RJ. Catalytic hydrolysis of vasoactive intestinal peptide by human autoantibody. Science. 1989;244:1158–62.
Paul S, Nishiyama Y, Planque S, Taguchi H. Theory of proteolytic antibody occurrence. Immunol Lett. 2006;103:8–16.
Paul S, Nishiyama Y, Planque S, Karle S, Taguchi H, Hanson C, et al. Antibodies as defensive enzymes. Springer Semin Immunopathol. 2005;26:485–503.
Planque S, Mitsuda Y, Taguchi H, Salas M, Morris MK, Nishiyama Y, et al. Characterization of gp120 hydrolysis by IgA antibodies from humans without HIV infection. AIDS Res Hum Retroviruses. 2007;23:1541–54.
Walsh DM, Selkoe DJ. Aβ oligomers—a decade of discovery. J Neurochem. 2007;101:1172–84.
Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–93.
Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid β peptide. Autoimmun Rev. 2008;7:415–20.
O'Nuallain B, Acero L, Williams AD, Koeppen HP, Weber A, Schwarz HP, et al. Human plasma contains cross-reactive Aβ conformer-specific IgG antibodies. Biochemistry. 2008;47:12254–6.
Taguchi H, Planque S, Nishiyama Y, Szabo P, Weksler ME, Friedland RP, et al. Catalytic antibodies to amyloid β peptide in defense against Alzheimer’s disease. Autoimmun Rev. 2008;7:391–7.
Taguchi H, Planque S, Nishiyama Y, Symersky J, Boivin S, Szabo P, et al. Autoantibody-catalyzed hydrolysis of amyloid β peptide. J Biol Chem. 2008;283:4714–22.
Qahwash IM, Boire A, Lanning J, Krausz T, Pytel P, Meredith SC. Site-specific effects of peptide lipidation on β-amyloid aggregation and cytotoxicity. J Biol Chem. 2007;282:36987–97.
Munch G, Mayer S, Michaelis J, Hipkiss AR, Riederer P, Muller R, et al. Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of β-amyloid peptide. Biochim Biophys Acta. 1997;1360:17–29.
Adekar SP, Klyubin I, Macy S, Rowan MJ, Solomon A, Dessain SK, et al. Inherent anti-amyloidogenic activity of human Ig γ heavy chains. J Biol Chem. 2009. doi:10.1074/jbc.M109.044321.
Silverman GJ, Goodyear CS. Confounding B-cell defenses: lessons from a staphylococcal superantigen. Nat Rev Immunol. 2006;6:465–75.
Paul S, Planque S, Zhou YX, Taguchi H, Bhatia G, Karle S, et al. Specific HIV gp120-cleaving antibodies induced by covalently reactive analog of gp120. J Biol Chem. 2003;278:20429–35.
Durova OM, Vorobiev II, Smirnov IV, Reshetnyak AV, Telegin GB, Shamborant OG, et al. Strategies for induction of catalytic antibodies toward HIV-1 glycoprotein gp120 in autoimmune prone mice. Mol Immunol. 2009;47:87–95.
Li Q, Gordon M, Cao C, Ugen K, Morgan D. Improvement of a low pH antigen-antibody dissociation procedure for ELISA measurement of circulating anti-Aβ antibodies. BMC Neurosci. 2007;8:22.
Mruthinti S, Buccafusco JJ, Hill WD, Waller JL, Jackson TW, Zamrini EY, et al. Autoimmunity in Alzheimer’s disease: increased levels of circulating IgGs binding Aβ and RAGE peptides. Neurobiol Aging. 2004;25:1023–32.
Weksler ME, Relkin N, Turkenich R, LaRusse S, Zhou L, Szabo P. Patients with Alzheimer’s disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37:943–8.
Istrin G, Bosis E, Solomon B. Intravenous immunoglobulin enhances the clearance of fibrillar amyloid-beta peptide. J Neurosci Res. 2006;84:434–43.
Relkin NR, Szabo P, Adamiak B, Burgut T, Monthe C, Lent RW, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer’s disease. Neurobiol Aging. 2009;30:1728–36.
Bacher M, Depboylu C, Du Y, Noelker C, Oertel WH, Behr T, et al. Peripheral and central biodistribution of (111)In-labeled anti-β-amyloid autoantibodies in a transgenic mouse model of Alzheimer’s disease. Neurosci Lett. 2009;449:240–5.
Mitsuda Y, Planque S, Hara M, Kyle R, Taguchi H, Nishiyama Y, et al. Naturally occurring catalytic antibodies: evidence for preferred development of the catalytic function in IgA class antibodies. Mol Biotechnol. 2007;36:113–22.
Taguchi H, Planque S, Sapparapu G, Boivin S, Hara M, Nishiyama Y, et al. Exceptional amyloid β peptide hydrolyzing activity of nonphysiological immunoglobulin variable domain scaffolds. J Biol Chem. 2008;283:36724–33.
St-Amour I, Laroche A, Bazin R, Lemieux R. Activation of cryptic IgG reactive with BAFF, amyloid β peptide and GM-CSF during the industrial fractionation of human plasma into therapeutic intravenous immunoglobulins. Clin Immunol. 2009;133:52–60.
Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–29.
Lacroix-Desmazes S, Bayry J, Kaveri SV, Hayon-Sonsino D, Thorenoor N, Charpentier J, et al. High levels of catalytic antibodies correlate with favorable outcome in sepsis. Proc Natl Acad Sci USA. 2005;102:4109–13.
Acknowledgments
This work was supported by the US National Institutes of Health (1R01AG025304). We thank the co-authors listed in our previous publications for their collaborations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, S., Planque, S. & Nishiyama, Y. Immunological Origin and Functional Properties of Catalytic Autoantibodies to Amyloid β Peptide. J Clin Immunol 30 (Suppl 1), 43–49 (2010). https://doi.org/10.1007/s10875-010-9414-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-010-9414-5