Abstract
Background
Subcutaneous immunoglobulin (SCIg) replacement therapy for primary immune deficiency disease (PIDD) is a safe, effective, and convenient alternative to intravenous Ig (IVIg) therapy. Although SCIg is typically administered weekly by infusion pump, administration by a rapid push technique may provide a greater degree of convenience. Rapid push administration has been an option at the author's clinic for several years. We report experience with a cohort of patients given a choice between rapid push and standard pump administration.
Methods
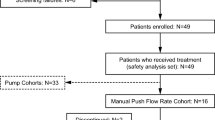
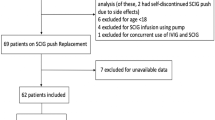
This was a retrospective chart review of PIDD patients at a single site who initiated treatment with SCIg (16% solution) therapy between January 1, 2006 and April 1, 2008. Patients selected either infusion pump or rapid push administration after receiving a description and demonstration of each method and training for home self-administration. Demographics, dose, adverse events (AEs), serum immunoglobulin G (IgG) levels, and therapy disposition (discontinued, switched technique) were recorded on a standardized data collection form.
Results
Charts for 104 patients (45 male, 59 female; mean age, 21.1 years; range, 0.5–67.6 years; SD = 17.9 years) were reviewed. Seventy-four patients (71%) chose rapid push. Mean SCIg dose was 32.11 g/month (range, 1.92–89.6 g/month; SD = 8.3 g/month) split into an average of 3.11 times per week. Volume per site ranged from 3 to 20 mL, typically administered over 5–20 min and at one site. Mean serum IgG levels did not differ significantly by administration method: pump, 1,153.06 mg/dL (SD = 240.8); rapid push, 1,225.8 mg/dL (SD = 299.8). Local infusion-site reactions were the most common AEs and were experienced by one third of patients in each group. Only two patients discontinued therapy because of an AE.
Conclusion
The results suggest that PIDD patients prefer SCIg administered as a rapid push rather than as conventional pump infusion. Serum IgG levels were comparable between methods, and safety was similar, if not slightly better, with rapid push. Rapid push offers the potential for even greater convenience than the pump infusion technique, although these results should be confirmed in prospective studies.





Similar content being viewed by others
References
Gardulf A. Treatment for primary antibody deficiencies. Advantages of the subcutaneous route. BioDrugs. 2007;21(2):105–16.
Moore ML, Quinn JM. Subcutaneous immunoglobulin replacement therapy for primary antibody deficiency: advancements into the 21st century. Ann Allergy Asthma Immunol. 2008;101(2):114–21.
Berger M. Principles of and advances in immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28(2):413–37.
Berger M. Subcutaneous IgG therapy in immune deficiency diseases. Clinical focus on primary immune deficiencies. Issue 13, February 2008. http://www.primaryimmune.org/publications/clinic_focus/cf_feb08.pdf. Accessed 28 April 2009.
Vivaglobin Immune Globulin Subcutaneous (Human) [package insert]. Marburg, Germany: CSL Behring GmbH; April 2007.
Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20(2):94–100.
Gardulf A, Nicolay U, Asensio O, Bernatowska E, Bock A, Costa Carvalho B, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies—a prospective, multi-national study. J Clin Immunol. 2006;26(2):177–85.
Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M, Subcutaneous IgG Study Group. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26(3):265–73.
Berger M, Duff K. Subcutaneous IgG replacement therapy. Updated 4/01/2007. http://www.uhhospitals.org/Portals/0/Docs/OurServices/Rainbow_Sub-Cu.pdf. Accessed 30 April 2009.
Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112(1):1–7.
Waniewski J, Gardulf A, Hammarström L. Bioavailability of γ-globulin after subcutaneous infusions in patients with common variable immunodeficiency. J Clin Immunol. 1994;14(2):90–7.
Kittner JM, Grimbacher B, Wulff W, Jager B, Schmidt RE. Patients' attitude to subcutaneous immunoglobulin substitution as home therapy. J Clin Immunol. 2006;26(4):400–5.
Gaspar J, Gerritsen B, Jones A. Immunoglobulin replacement treatment by rapid subcutaneous infusion. Arch Dis Child. 1998;79(1):48–51.
Gardulf A, Nicolay U, Asensio O, Bernatowska E, Bock A, Costa-Carvalho BT, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114(4):936–42.
Fasth A, Nystrom J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol. 2008;28(4):370–8.
Acknowledgements
Financial support for this retrospective analysis was provided by CSL Behring (King of Prussia, PA, USA). The author takes full responsibility for the content and confirms that it reflects his viewpoint and medical expertise. He wishes to acknowledge Sandra Westra, PharmD, of Churchill Communications (Maplewood, NJ, USA) for providing editorial assistance; Frank J. Rodino, PA, MHS, of Churchill Clinical Research for protocol development, data collection, and data analysis; and Kristin Epland, FNP, of Midwest Immunology Clinic for data collection. CSL Behring did not influence the content of the manuscript nor did the author receive financial compensation for writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapiro, R. Subcutaneous Immunoglobulin Therapy by Rapid Push is Preferred to Infusion by Pump: A Retrospective Analysis. J Clin Immunol 30, 301–307 (2010). https://doi.org/10.1007/s10875-009-9352-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-009-9352-2