Abstract
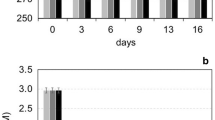
We examined the effect of warming on microbes in coastal waters in temperate and subtropical zones, Suruga Bay in Japan and Ha Long Bay in Viet Nam, respectively. Incubation of intact communities (500 mL each) at temperatures three or five degrees higher than the in-situ temperatures enhanced both the abundance and growth rate of prokaryotes. We also conducted size fractionation and dilution experiments to elucidate whether this behavior was the result of an increased rate of reproduction (“bottom-up” effect) or reduced mortality (“top-down” effect). For two of the five cases analyzed, enhancement of the growth rate could be ascribed to the bottom-up effect of the warming. For two other cases it could be ascribed to the top-down effect, irrespective of whether or not warming enhanced the bottom-up effect. The effect of warming was not clearly apparent for prokaryotes of subtropical coastal waters, where the in-situ temperature was nearly 30 °C. Among the prokaryotes, Gammaproteobacteria tended to predominate as a result of warming. These results suggest that warming may affect microbial growth even in subtropical coastal waters in some seasons and that the response of Gammaproteobacteria to warming of temperate and subtropical coastal waters is among the most rapid.





Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman JD (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Bouvy M, Bettarel Y, Bouvier C, Domaizon I, Jacquet S, Le Floc’h E, Montanie H, Mostajir B, Sime-Ngando T, Torreton JP, Vidussi F, Bouvier T (2011) Trophic interactions between viruses, bacteria and nanoflagellates under various nutrient conditions and simulated climate change. Environ Microbiol 13(7):1842–1857
Brown JR, Volker C (2004) Phylogeny of gamma-proteobacteria: resolution of one branch of the universal tree? BioEssays 26(5):463–468
Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska ES, Brander KM, Brown C, Bruno JF, Duarte CM, Halpern BS, Holding J, Kappel CV, Kiessling W, O’Connor MI, Pandolfi JM, Parmesan C, Schwing FB, Sydeman WJ, Richardson AJ (2011) The pace of shifting climate in marine and terrestrial ecosystems. Science 334(6056):652–655
Colwell RR (1996) Global climate and infectious disease: the cholera paradigm. Science 274:2025–2031
Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci USA 106(31):12788–12793
Giovannoni SJ, DeLong EF, Olsen GJ, Pace NR (1988) Phylogenetic group specific oligodeoxynucleotide probes for identification of single microbia cells. J Bacteriol 170:720–726
Hao DM, Tashiro T, Kato M, Sohrin R, Ishibashi T, Katsuyama C, Nagaosa K, Kimura H, Thanh TD, Kato K (2010) Population dynamics of Crenarchaeota and Euryarchaeota in the mixing front of river and marine waters. Microbes Environ 25(2):126–132
IPCC (2013) Summary for policymakers. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V and Midgley PM (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jiang Z, Liub J, Chen J, Chen Q, Yan X, Xuan J, Zeng J (2014) Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during the past 50 years. Water Res 54:1–11
Kato K, Stabel HH (1984) Studies on the carbon flux from phyto- to bacterioplankton communities in Lake Constance. Arch Hydrobiol 102:177–192
Kogure K, Simidu U, Taga N (1980) Effect of phyto- and zooplankton on the growth of marine bacteria in filtered seawater. Bull Jpn Soc Sci Fish 46:323–326
Landry MR, Hassett RP (1982) Estimating the grazing impact of marine micro-zooplankton. Mar Biol 67:283–288
Lane JD (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goofellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lara E, Arrieta JM, Garcia-Zarandona I, Boras JA, Duarte CM, Agustí S, Wassmann PF, Vaqué D (2013) Experimental evaluation of the warming effect on viral, bacterial and protistan communities in two contrasting Arctic systems. Aquat Microb Ecol 70:17–32
Lim EL, Amaral LA, Caron DA, Delong EF (1993) Application of rRNA-based probes for observing marine nanoplanktonic protists. Appl Environ Microbiol 59:1647–1655
Lim EL, Caron DA, Delong EF (1996) Development and field application of a quantitative method for examining natural assemblages of protists with oligonucleotide probes. Appl Environ Microbiol 62:1416–1423
Lomas MW, Glibert P, Shiah F, Smith EM (2002) Microbial processes and temperature in Chesapeake Bay: current relationships and potential impacts of regional warming. Glob Change Biol 8:51–70
Madigan MT, Martinko JM, Parker J (2000) Brock biology of microorganisms, 9th edn. Prentice-Hall Inc, New Jersey, pp 455–484
Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH (1992) Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol 15:593–600
Oliver JD, Pruzzo C, Vezzulli L, Kaper JB (2013) Vibrio species. In: Doyle MP, Buchanan RL (eds) Food microbiology: fundamentals and frontiers, 4th edn. ASM, Washington, pp 401–440
Petchey OL, McPhearson PT, Casey TM, Morin PJ (1999) Environmental warming alters food-web structure and ecosystem function. Nature 402:69–72
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25(5):943–948
Sarmento H, Montoya JM, Vázquez-Domínguez E, Vaqué D, Gasol JM (2010) Warming effects on marine microbial food web processes: how far can we go when it comes to predictions? Philos Trans R Soc Lond B Biol Sci 365(1549):2137–2149
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Shiah F, Ducklow HW (1994) Temperature and substrate regulation of bacterial abundance, production and specific growth rate in Chesapeake Bay, USA. Mar Ecol Prog Ser 103:297–308
Sjöstedt J, Hagström Å, Zweifel UL (2012) Variation in cell volume and community composition of bacteria in response to temperature. Aquat Microb Ecol 66(3):237–246
Sogin ML, Gunderson JH (1987) Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann N Y Acad Sci 503:125–139
Šolić M, Krstulović N (1994) Role of predation in controlling bacterial and heterotrophic nanoflagellate standing stocks in the coastal Adriatic Sea: seasonal patterns. Mar Ecol Prog Ser 114:219–235
Šolić M, Krstulović N, Vilibić I, Bojanić N, Kušpilić G, Šestanović S, Šantić D, Ordulj M (2009) Variability in the bottom-up and top-down control of bacteria on trophic and temporal scale in the middle Adriatic Sea. Aquat Microb Ecol 58:15–29
Somerville CC, Knight IT, Straube WL, Colwell RR (1989) Simple, rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol 55:548–554
Takenaka T, Tashiro T, Ozaki A, Takakubo H, Yamamoto Y, Maruyama T, Nagaosa K, Kimura H, Kato K (2007) Planktonic bacterial population dynamics with environmental changes in coastal areas of Suruga Bay. Microbes Environ 22(3):257–267
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsai AY, Chiang KP, Chang J, Gong GC (2008) Seasonal variations in trophic dynamics of nanoflagellates and picoplankton in coastal waters of the western subtropical Pacific Ocean. Aquat Microb Ecol 51:263–274
Tsai AY, Gong GC, Hung J (2013) Seasonal variations of virus- and nanoflagellate-mediated mortality of heterotrophic bacteria in the coastal ecosystem of subtropical western Pacific. Biogeosciences 10:3055–3065
Vezzulli L, Brettar I, Pezzati E, Reid PC, Colwell RR, Hofle MG, Pruzzo C (2012) Long-term effects of ocean warming on the prokaryotic community: evidence from the Vibrios. ISME J 6(1):21–30
Vezzulli L, Colwell RR, Pruzzo C (2013) Ocean warming and spread of pathogenic vibrios in the aquatic environment. Microb Ecol 65:817–825
von Scheibner M, Dӧrge P, Biermann A, Sommer U, Hoppe HG, Jürgens K (2013) Impact of warming on phyto-bacterioplankton coupling and bacterial community composition in experimental mesocosms. Environ Microbiol 16(3):718–733
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
White PA, Kalff J, Rasmussen JB, Gasol JM (1991) The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microb Ecol 21:99–118
Wright RT, Coffin RB (1983) Planktonic bacteria in estuaries and coastal waters of northern Massachusetts: spatial and temporal distribution. Mar Ecol Prog Ser 11:205–216
Yokokawa T, Nagata T (2005) Growth and grazing mortality rates of phylogenetic groups of bacterioplankton in coastal marine environments. Appl Environ Microbiol 71(11):6799–6807
Yokokawa T, Nagata T, Cottrell MT, Kirchman DL (2004) Growth rate of the major phylogenetic bacterial groups in the Delaware estuary. Limnol Oceanogr 49(5):1620–1629
Acknowledgments
We thank editors and two anonymous reviewers for their careful reading the manuscript. This study was supported by the Graduate School of Science and Technology, Shizuoka University; Special Research Fund for Future Program of Shizuoka University, Japan; and project VAST03.06/13-14, Viet Nam. We thank all laboratory members for their assistance in sampling and in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tuyet, D.T.A., Tanaka, T., Sohrin, R. et al. Effects of warming on microbial communities in the coastal waters of temperate and subtropical zones in the Northern Hemisphere, with a focus on Gammaproteobacteria . J Oceanogr 71, 91–103 (2015). https://doi.org/10.1007/s10872-014-0264-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-014-0264-2