Abstract
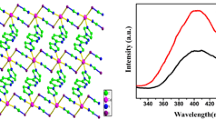
A new zinc(II) complex [Zn(L1)Cl2]‧CH3CN‧1/2H2O (complex 1), where L1 stands for 2,6-Bis{[(2-ethylphenyl)imino]ethyl}pyridine, has been synthesized and characterized. Both the crystal structures of complex 1 and L1 have been determined by single-crystal X-ray diffraction. L1 crystallizes in monoclinic system P21/n space group, with a = 7.7165(5) Å, b = 9.1139(7) Å, c = 30.535(2) Å, β = 96.037(2)° and Z = 4. Complex 1 crystallizes in the orthorhombic system, space group Pbca, and its crystallographic data are a = 14.0520(9) Å, b = 14.5780(11) Å, c = 26.3450(17) Å, α = β = γ = 90°, and Z = 8. In complex 1, Zn1 atom is five-coordinated with a trigonal bipyramidal geometry. L1 acts as a tridentate ligand and coordinated to Zn1 with three nitrogen atoms. A two-dimensional supermolecular network is formed by hydrogen bonds, in which acetonitrile and water solvent molecules play an important role. The luminescent properties of L1 and complex 1 were investigated in the solid state. The results indicate that when coordinated with Zn atom, the fluorescence intensity of complex 1 increases accompanied by a blue-shift, which can be attributed to metal perturbed π–π* intraligand fluorescence.
Graphic Abstract
The single crystals of 2,6-Bis{[(2-ethylphenyl)imino]ethyl}pyridine (L1) and it’s Zinc(II) complex [Zn(L1)Cl2]‧CH3CN‧1/2H2O (1) were determined. In the crystal structure of complex 1, the acetonitrile and water solvent molecules play great role in the formation of hydrogen bonds, forming a two-dimensional supermolecular network structure.










Similar content being viewed by others
References
Zhang Y, Yuan S, Day G, Wang X, Yang X, Zhou HC (2018) Coord Chem Rev 354:28
Chen DM, Tian JY, Chen M, Liu CS, Du M (2016) ACS Appl Mater Inter 8:18043
Yao RX, Cui X, Jia XX, Zhang FQ, Zhang XM (2016) Inorg Chem 55:9270
Das D, Chand BG, Dinda J, Sinha C (2007) Polyhedron 26:555
Shao KZ, Zhao YH, Wang XL, Lan YQ, Wang DJ, Su ZM, Wang RS (2009) Inorg Chem 48:10
Sazanovich LV, Kirmaier C, Hindin E, Yu L, Bocian DF, Lindsey JS, Holten D (2004) J Am Chem Soc 126:2664
Jennifer LB, Simon BB (2012) Heterocycles 84:1313
Chen YF, Qian CT, Sun J (2003) Organometallics 22:1231
Gong DR, Jia XY, Wang BL, Wang F, Zhang CY, Zhang XQ, Jiang LS, Dong WM (2011) Inorg Chim Acta 373:47
Zhang D, Zhang YL, Hou WJ, Guan ZB, Huang Z (2017) Organometallics 36:3758
Fan RQ, Wang P, Yang YL, Zhang YJ, Yin YB, Hasi W (2010) Polyhedron 29:2862
Kim JK, Yoo H (2012) J Struc Chem 53:347
Shaban SY, Ramadan AM, van Eldik R (2012) J Coord Chem 65:2415
Ghedini M, Pucci D, Crispini A, Bellusci A, La Deda M, Aiello I, Pugliese T (2007) Inorg Chem Commun 10:243
Lv CN, Zhang L, Li JX, Hu JS, Shi JJ, Guo YC (2019) Inorg Chem Commun 105:240
Gibson VC, Redshaw C, Solan GA (2007) Chem Rev 107:1745
Sheldrick GM (2008) Acta Cryst A 64:112
Sheldrick GM (2015) Acta Cryst A 71:3
Su BY, Feng GX (2010) Polym Int 59:1058
Wang M, Guo G (2016) Chem Commun 52:13194
Acknowledgments
We gratefully acknowledge the financial supports of the Foundation of Hubei Educational Committee (No. D20172904).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, W. Synthesis, Characterization and Fluorescence Property of a New Zinc(II) Complex Based on 2,6-Bis(imino)pyridyl Ligand. J Chem Crystallogr 51, 132–138 (2021). https://doi.org/10.1007/s10870-020-00828-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-020-00828-3