Abstract
Analysis of the crystal packing of the title porphyrin derivative (C72H54N4O4) suggests no classical hydrogen bonds between neighbor molecules. X-ray crystal structure shows that all benzyl units of this porphyrin have close C–H⋯π weak contacts with phenyl or porphyrinyl units forming a network of porphyrin rings. Also C–H⋯O and parallel aromatic–aromatic weak interactions play an important role in structure extension. All of these interactions control the crystal packing of molecules. X-ray diffraction was used to perform single crystal analysis. The structure was solved in the triclinic space group P-1, with unit cell parameters: a = 8.0597(3) Å, b = 11.6862(4) Å, c = 14.2572(5) Å, α = 96.173(3)°, β = 93.150(4)°, and γ = 93.679(3)°, V = 1329.72(8) Å3, Z = 1.
Graphical abstract
The lack of strong intermolecular hydrogen bonds and presence of numerous weak hydrogen bonds are decisive factors in crystal structure of the examined meso-tetrakis[4-(benzyloxy)-phenyl]porphyrin.

Similar content being viewed by others
Introduction
The color of porphyrins derived from a characteristic central ring results from the electronic properties of these compounds. Particular fragments present in the large central ring cause only negligible deviation from planarity, due to electron coupling in the central moiety, and this was unequivocally confirmed by X-ray analysis. Crystallographic structures of many porphyrin derivatives and their complexes have been determined e.g. [1,2,3,4,5,6]. Such studies may not be possible in case of some porphyrins as they may also occur as amorphous substances. This problem occurs most frequently in case of unsymmetric porphyrins.
Our studies, which have been concerned with synthesis of novel tetraphenylporphyrin derivatives, led to many such compounds. The majority of them did not yield monocrystals suitable for crystallographic studies. We also attempted to investigate the structure of amorphous asymmetric porphyrins using synchrotron radiation [7, 8]. Such studies are burdened by severe inconveniences such as access to suitable source of radiation or handling of experimental results.
X-ray study data (CSD) made possible to assess the existence as well as possible value of non-covalent π–π or C–H⋯π interactions between aromatic rings [9,10,11].
The last few decades noted increased interest in C–H⋯π and π⋯π interactions of inter- or intramolecular type, especially in crystal structures, as judged from published reports, e.g. [12,13,14,15,16,17,18,19,20] for C–H⋯π interactions and [10, 21,22,23,24,25,26,27,28] for π⋯π interactions as well as references cited therein. Interactions of this type are especially important for structures described in supramolecular chemistry texts. A very well written overview of such interactions can be found in [29]. Nevertheless, premises underlying their alleged importance in crystallographic structure formation are not entirely clear [30].
These papers include a vast number of references concerning weak hydrogen bonds in crystals formed by various compounds. An essential criterion for occurrence and strength of these bonds are distances between hydrogen atom (in C–H) and the plane of aromatic ring [less than 2.9 Å (sum of van der Waals distances)] as well C–H⋯π access angles (varied from 140°–180°) [13]. Other criteria were adopted for parallel aromatic–aromatic interactions (π–π interactions). Aromatic rings should lie in planes parallel to each other (angle between planes must be less than 10°). Such planes should not be separated by a distance greater than 4 Å, and the distance between centers of aromatic rings should not exceed 6 Å [9].
In the molecule reported herein one could clearly discern such type of interactions and their effect on localization of porphyrin molecules in the crystal structure.
Experimental
1H NMR spectra were recorded in CDCl3 using a 400 MHz Varian spectrometer. The peaks were referenced to the residual CHCl3 resonances in 1H (7.26 ppm). IR spectra were recorded using Bio-Rad FTS-600 with the samples in the form of KBr pellets. UV–Vis spectra were recorded in chloroform solution using a Genesys 6 (ThermoSpectronic) spectrophotometer. The single crystal X-ray experiments were performed at 298 K. The data were collected using an Oxford Diffraction kappa diffractometer with a Sapphire3 CCD detector (Oxford Diffraction Ltd., Yarnton, UK). To integrate collected data the CrysAlis PRO software (version 1.171.35.21b, Agilent Technologies) was used. The structure was solved using direct methods with the SHELXS97 software and the solutions were refined using SHELXL97 program [31]. Hydrogen positions were calculated according to geometrical criteria and refined isotropically. All others atoms were refined anisotropically. Crystal data, selected geometric parameters and selected hydrogen-bond parameters are summarized in Tables 1, 2 and 3, respectively.
CCDC 1510250 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viahttp://www.ccdc.ac.uk/data_request/cif.
meso-Tetrakis[4-(benzyloxy)phenyl]porphyrin (C72H54N4O4) was synthesized by condensation of 4-benzyloxybenzaldehyde and pyrrole in propionic acid according to [32, 33]. Violet-colored crystals were obtained using column chromatography of crude product with chloroform and by slow evaporation of concentrated eluent.
MS (ESI): m/z 1039.5 [M + H]+, (calcd. for [M + H]+ 1039.4).
1H NMR (400 MHz, DMSO-d6): δH, ppm 8.89 (s, 8H), 8.15 (d, 8H), 7.68 (d, 8H), 7.53 (t, 8H), 7.45 (t, 4H), 7.39 (d, 8H), 5.39 (s, 8H), -2.73 (bs, 2H).
UV–vis (CHCl3): λmax, nm (log ε) 421 (4.51), 518 (3.38), 555 (3.25), 593 (3.01), 650 (3.06).
IR (KBr): ν, (cm−1) 3331, 3119, 3033, 2861, 1602, 1503, 1453, 1410, 1390, 1344, 1287, 1218, 1172, 1107, 1081, 964, 928, 912, 878, 856, 834, 805, 786, 735, 695, 642, 618, 590, 556, 530, 501, 455, 422.
Results and discussion
We report here the crystallographic structure of an asymmetric meso-tetrakis[4-(benzyloxy)phenyl]porphyrin (Scheme 1). This compound has been known since 1968 [34]. It was prepared using Adler-Longo method (9–10% yield). Twenty years later the same compound was obtained by a method devised and named after Lindsey, with 8–40% yield [35, 36]. We noticed interesting molecular interactions while investigating the structure of this compound. Despite being weak they can affect (or affect) localization of porphyrin rings in the crystal space. In porphyrin molecules there are no functional groups capable of forming strong intermolecular hydrogen bonds. The only suitable moieties are found inside the molecular ring but they are “isolated” by the remaining fragments of the porphyrin ring and its substituents.
Porphyrin 1 molecule is centrosymmetric with two sets of benzyloxyphenyl units orientation (Fig. 1). Central core of the porphyrin ring is approximately planar with r.m.s deviation of fitted atoms equal to 0.05 Å. The angles between substituent benzene rings and the porphyrin core plane are 61.79(4)° and 65.78(6)°. Besides intramolecular hydrogen N–H⋯N bonds inside the porphyrin core only intermolecular weak hydrogen bonds are present in this molecule. Detailed information regarding intramolecular hydrogen bonds in the molecules of 1 is given in Table 3.
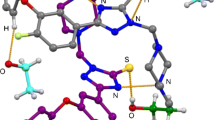
Packing of meso-tetrakis[4-(benzyloxy)phenyl]porphyrin 1 (Fig. 2) is influenced by few weak C–H⋯π, C–H⋯O and face-to-face benzene rings intermolecular contacts forming chains of molecules (in zig-zag forms) parallel to (111). The C–H⋯π contacts form the exclusive contribution to the packing, involving two para—hydrogen atoms from two benzyloxy groups attached to phenyls in 5 and 15 position of porphyrin ring, respectively (Fig. 3a), and two meta - hydrogen atoms from two benzyloxy groups attached to phenyls in 10 and 20 position of the porphyrin ring, respectively (Fig. 3b). Analysis of these C–H⋯π interactions gave C–H⋯centroid phenyl distances of 2.727 Å (H⋯Centroid)/3.628 Å (C⋯Centroid), and C–H⋯centroid porphyrin ring distances of 2.676 Å (H⋯Centroid)/3.523 Å (C⋯Centroid), with corresponding C–H⋯centroid phenyl ring angle of 161° and C–H⋯centroid porphyrin ring angle of 152°, for para and meta interactions, respectively (Fig. 3a, b). The geometrical categories according to [37] are: both C–H⋯π interactions is Type I (for C–H⋯πar α = 163.26°, Θ = 83–91°; for C–H⋯πpyr α = 151.87°, Θ = 92°).
a Illustration of C–H⋯π hydrogen bonds between two benzyloxyphenyl units of porphyrins located in A and G positions. Substituents in other meso positions of porphyrins were omitted for clarity. b Hydrogen bond motifs between hydrogen atom of benzene unit and porphyrin core neighbor molecule viewed parallel to the direction of porphyrin rings (in H and F positions; similar situation as in B and D positions). Dashed lines represent hydrogen bonds. c Exemplary illustration of C–H⋯O hydrogen bonds between two benzyloxyphenyl units located in A and C positions (similar to E and G positions). Substituents in 10- and 20-positions of porphyrins were omitted for clarity. d Illustration of both C–H⋯π weak hydrogen bonds between two benzyloxyphenyl units and C–H⋯O hydrogen bonds between oxygen atom of benzyloxyphenyl unit and β-hydrogen of porpyrin ring between three molecules of porphyrins located in A, B and G positions. Substituents of 10,15,20-positions of all porphyrins were omitted for clarity
This (C–H⋯π) interaction for rings attached in positions 5 and 15 of the porphyrin 1 causes the two benzene rings of the adjacent molecules’ benzyl fragment to be positioned parallel to each other in planes 3.560(1) Å away and centroid–centroid distance of 3.890 Å. Mutual shift of the rings with respect to each other (horizontal displacement of parallel phenyl rings) is ca. 1.38 Å. In [9, 38] the authors calculated the energy of benzene rings’ interaction in their various orientations. They concluded that the interaction between two parallel benzene molecules occurs if centroid–centroid distance < 6.0 Å and the distance between the planes of the interacting benzene molecules is < 4.0 Å.
With optimum separation distance of both rings’ planes for the above-described parameters the energy of interaction is ca. − 2.8 kcal/mol (almost the maximum energy for aromatic–aromatic interactions acc. to [9]).
Similar situation occurs between phenyl rings attached directly to porphyrin rings in positions 10 and 20 (benzyl rings participate in forming C–H⋯π bond of the porphyrin ring). Mutual positioning and shift of phenyl rings are similar in positions both 5 and 15, yet the distance between the ring planes and their centroid–centroid distance are greater (ca. 4.15 Å and ca. 4.4 Å, respectively) (Fig. 3b). These values remain within the range of benzene rings’ interaction. The energy of these interactions, although considerably lower, also affects the formation of the molecular system spatial structure.
Another weak contact is C–H⋯O hydrogen bond between one of the β hydrogen atoms of the porphyrin pyrrole rings and oxygen atoms of the ether bridge between adjacent molecules. The length of these bonds is 2.675(H⋯O) / 3.485 (C⋯O) Å, whereas α angle is ca. 146°. This interaction is shown in Fig. 3c.
Torsional angles between porphyrin ring and phenyl rings directly linked to it are: ± 59.87° and ± 65.36° for rings in positions 5 and 15 as well as in positions 10 and 20, respectively. Comparing these values leads to the conclusion that deviation of these phenyl rings from the plane of porphyrin ring is affected to a greater degree by hydrogen bonds formed between substituents in positions 5 and 15 than those formed between substituents in positions 10 and 20, respectively. These deviations are not dramatically greater, however, than those occurring in tetraphenylporphyrin (61.0° and 63.1°, respectively) [39].
Distances between planes of porphyrin ring planes are 3.560 Å. Mutual positioning of porphyrin molecules in the crystal is similar to that in porphyrin solutions that do not contain fragments capable of forming strong hydrogen bonds. Weak hydrogen bonds lead to the formation of porphyrin H-aggregates (face-to-face type) in solution, as opposed to J-aggregates (head-to-tail type) formed by porphyrins with strong hydrogen bonds between molecules in solution [40].
Conclusions
Attachment of benzyloxy fragments in para position of TPP phenyl rings makes possible additional interactions involving molecules of the starting compound. Such additional interactions accompany formation of crystallographic structures and involve particular porphyrin molecules. The interactions cause the two benzyl rings to move closer (face-to-face), with the distance between parallel planes of both rings going down to 3.6 Å, and with centroid–centroid distance equal to 3.89 Å. Similar positioning of aromatic rings with respect to each other occurs between phenyl rings directly attached to porphyrin at positions 10 and 20. In this case, however, the distances between the planes of aromatic moieties are greater by ca. 0.5 Å which weakens to a certain degree the π–π interaction of these fragments. Overall, they must contribute to the formation of the crystallographic structure described herein. As exemplified by the fragments of this structure, despite its obvious complexity it is the lack of strong hydrogen bonds and presence of numerous (but weak) hydrogen bonds (the very existence and impact on crystal structure of which continue to be debated) that clearly are decisive factors in spatial structure formation of the examined crystal.
References
Walawalkar MG, Nethaji M, Krishnan V (1993) Acta Cryst C49:479
Dastidar P, Goldberg I (1996) Acta Cryst C52: 1976
Balaban TS, Eichhöfer A, Lehn J-M (2000) Eur J Org Chem 2000(24):4047
Diskin-Posner Y, Patra GK, Goldberg I (2002) Acta Cryst C58:m344
Zhao H-B, Chen L, Wang B-Y, Liao J-X, Xu Y-J (2013) Acta Cryst C69:651
Lenarska A, Zubko M, Kuś P, Kusz J, Ratuszna A (2012) Acta Cryst E 68:o2797
Pasewicz A, Idziak D, Koloczek J, Kuś P, Wrzalik R, Fennell T, Honkimäki V, Ratuszna A, Burian A (2008) J Mol Struct 875:167
Idziak D, Pasewicz A, Kołoczek J, Kuś P, Wrzalik R, Fennell T, Honkimäki V, Ratuszna A, Burian A (2007) Chem Phys Lett 446:36
Ninković DB, Janjić GV, Veljković D, Sredojević DN, Zarić SD (2011) ChemPhysChem 12:3511
Grimme S (2008) Angew Chem Int Ed 47:3430
Główka ML, Martynowski D, Kozłowska K (1999) J Mol Struct 474:81
Umezawa Y, Tsuboyama S, Honda K, Uzawa J, Nishio M (1998) Bull Chem Soc Jpn 71:1207
Takahashi O, Kohno Y, Iwasaki S, Saito K, Iwaoka M, Tomoda S, Umezawa Y, Tsuboyama S, Nishio M (2001) Bull Chem Soc Jpn 74:2421
Jennings WB, Farrell BM, Malone JF (2001) Acc Chem Res 34:885
Desiraju GR (2002) Acc Chem Res 35:565
Noshio M (2004) CrystEngComm 6:130
Tsuzuki S (2012) Annu Rep Prog Chem Sect C:PhysChem 108:69
Desiraju GR, T.Steiner (1999) The weak hydrogen bond in structural chemistry and biology. IUCr Monographs on Crystallography. Oxford Science Publications, Oxford
Plevin MJ, Bryce DL, Boisbouvier J (2010) Nature Chem 2:466
Ribas J, Cubero E, Luque FJ, Orozco M (2002) J Org Chem 67:7057
Hunter CA, Sanders JKM (1990) J Am Chem Soc 112:5525
McGaughey GB, Gagné M, Rappé AK (1998) J Biol Chem 273:15458
Mignon P, Loverix S, Steyaert J, Geerlings P (2005) Nucleic Acid Res 33:1779
Sinnokrot MO, Sherrill CD (2006) J Phys Chem A 110:10656
Podeszwa R, Bukowski R, Szalewicz K (2006) J Phys Chem A 110:10345
Park YC, Lee JS (2006) J Phys Chem A 110:5091
Martinez CR, Iverson BL (2012) Chem Sci 3:2191
Marjo CE, Bishop R, Craig DC, O’Brien A, Scudder ML (1994) J Chem Soc Chem Commun 21:2513
Mayer EA, Castellano RK, Diederich F (2003) Angew Chem Int Ed 42:1210
Taylor R (2016) CrystGrowth Des 16:4165
Sheldrick GM (2008) Acta Cryst A64:112
Little RG, Anton JA, Loach PA, Ibers JA (1975) J Heterocycl Chem 12:343
Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L (1967) J Org Chem 32:476
Samuels E, Shuttleworth R, Stevens TS (1968) J Chem Soc C Org. https://doi.org/10.1039/J39680000145
Lindsey JS, Hsu HC, Schreiman IC (1986) Tetrahedron Lett 27:4969
Lindsey JS, Schreiman IC, Hsu HC, Kearney PC, Marquerettaz AM (1987) J Org Chem 52:827
Malone JF, Murray CM, Charlton MH, Docherty R, Lavery AJ (1997) J Chem Soc Faraday Trans 93:3429
Lee EC, Kim D, Jurečka P, Tarakeshwar P, Hobza P, Kim KS (2007) J Phys Chem A 111:3446
Silvers S, Tulinsky A (1964) J Am Chem Soc 86:927
Villari V, Mineo P, Scamporrino E, Micali N (2012) RSC Adv 2:12989
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuś, P., Kusz, J. & Książek, M. Aromatic C–H⋯π, C–H⋯O and parallel aromatic–aromatic interactions in the crystal structure of meso-tetrakis[4-(benzyloxy)phenyl]porphyrin. J Chem Crystallogr 50, 21–27 (2020). https://doi.org/10.1007/s10870-018-0752-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0752-0