Abstract
Here, we compare structures determined by X-ray diffraction and subsequent Hirshfeld surface analysis to identify and understand the non-covalent interactions within the lattices of chromone, 6-methylchromone, 6-methoxychromone, 6-fluorochromone, and 6-chlorochromone with reported 6-bromochromone. In chromone, H-bonds and CH–л interactions predominate. H-bonds and aryl-stacking interactions are distinct in 6-methylchromone and 6-methoxychromone. The 6-fluorochromone, showed two types of H-bonds with O···H bonds having a greater contribution than F···H. In contrast, 6-chlorochromone and 6-bromochromone, the halogen contributes the larger percentage of stabilizing H-bonding with Cl···H and Br···H predominating over the O···H bonds. Compound 1 crystallizes in the monoclinic space group P21 /n with a = 8.1546(8) Å, b = 7.8364(7) Å, c = 11.1424(11) Å, β = 108.506(2)° and Z = 4. Compound 2 crystallizes in the triclinic space group P-1 with a = 7.0461(3) Å, b = 10.2108(5) Å, c = 10.7083(5) Å, α = 89.884(2)°, β = 77.679(2)°, γ = 87.367(2)° and Z = 4. Compound 3 crystallizes in the monoclinic space group P21/n with a = 8.1923(4) Å, b = 7.0431(3) Å, c = 15.3943(8) Å, β = 92.819(2)° and Z = 4. Compound 4 crystallizes in the triclinic space group P1 with a = 3.7059(2) Å, b = 6.1265(4) Å, c = 7.6161(5) Å, α = 84.085(3)°, β = 87.070(3)°, γ = 83.390(3)° and Z = 1. Compound 5 crystallizes in the monoclinic space group P2 1 with a = 3.8220(2) Å, b = 5.6985(2) Å, c = 16.9107(7) Å, β = 95.8256(18)° and Z = 2.
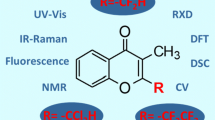
Graphical Abstract
The effect of substituents at the 6-position on chromone on their crystal structures using Hirshfeld surface and fingerprint analysis.










Similar content being viewed by others
References
Desiraju GR (2013) J Am Chem Soc 135(27):9952–9967
Tiekink ERT (2012) Crystal engineering. Supramolecular chemistry. Wiley, New York
Aakeroy CB, Champness NR, Janiak C (2010) CrystEngComm 12(1):22–43
Desiraju GR (2007) Angew Chem Int Ed 46(44):8342–8356
Braga D, Brammer L, Champness NR (2005) CrystEngComm 7(1):1–19
Hollingsworth MD (2002) Science 295(5564):2410–2413
Braga D, Desiraju GR, Miller JS, Orpen AG, Price SL (2002) CrystEngComm 4(83):500–509
Stadler A-M, Lehn J-MP (2014) J Am Chem Soc 136(9):3400–3409
Dolain C, Maurizot V, Huc I (2003) Angew Chem Int Ed 42(24):2738–2740
Kay ER, Leigh DA, Zerbetto F (2007) Angew Chem Int Ed 46(1–2):72–191
Lehn JM (2006) Molecular and supramolecular devices. Supramolecular chemistry. Wiley, New York, pp 89–138
Tian J, Thallapally PK, McGrail BP (2012) Gas storage and separation in supramolecular materials. Supramolecular chemistry. Wiley, New York
Makal TA, Li J-R, Lu W, Zhou H-C (2012) Chem Soc Rev 41(23):7761–7779
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su C-Y (2014) Chem Soc Rev 43(16):6011–6061
Wu C-D (2011) Crystal engineering of metal-organic frameworks for heterogeneous catalysis. Selective nanocatalysts and nanoscience. Wiley, New York, pp 271–298
Aakeröy CB, Beatty AM (2001) Aust J Chem 54(7):409–421
Desiraju GR (1989) Crystal engineering: the design of organic solids, vol 54. Elsevier, Amsterdam
Verpoorte R, Memelink J (2002) Curr Opin Biotechnol 13(2):181–187
Keri RS, Budagumpi S, Pai RK, Balakrishna RG (2014) Eur J Med Chem 78:340–374
Gaspar A, Matos MJ, Garrido J, Uriarte E, Borges F (2014) Chem Rev 114(9):4960–4992
Ishar MPS, Singh G, Singh S, Sreenivasan KK, Singh G (2006) Bioorg Med Chem Lett 16(5):1366–1370
Sakamoto M, Yagishita F, Kanehiro M, Kasashima Y, Mino T, Fujita T (2010) Org Lett 12(20):4435–4437
Sakamoto M, Kanehiro M, Mino T, Fujita T (2009) Chem Commun 17:2379–2380
Hanifin JW, Cohen E (1969) J Am Chem Soc 91(16):4494–4499
Hanifin JW, Cohen E (1966) Tetrahedron Lett 7(44):5421–5426
Salpage SR, Donevant LS, Smith MD, Bick A, Shimizu LS (2016) J Photochem Photobiol A 315:14–24
Schmidt GMJ (1971) Pure Appl Chem 27:647–678
Cohen MD, Schmidt GMJ, Sonntag FI (1964) J Chem Soc 384:2000–2013
Cohen MD, Schmidt GMJ (1964) J Chem Soc 383:1996–2000
Spackman MA, McKinnon JJ, Jayatilaka D (2008) CrystEngComm 10(4):377–388
Parkin A, Barr G, Dong W, Gilmore CJ, Jayatilaka D, McKinnon JJ, Spackman MA, Wilson CC (2007) CrystEngComm 9(8):648–652
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Chem Commun 37:3814–3816
Spackman MA, McKinnon JJ (2002) CrystEngComm 4(66):378–392
McKinnon JJ, Mitchell AS, Spackman MA (1998) Chem Eur J 4(11):2136–2141
Spackman MA, Jayatilaka D (2009) CrystEngComm 11(1):19–32
Staples RJ, Lea W (2005) New Cryst Struct 220(3):371–372
SMART Version 5.631, SAINT+ Version 6.45a (2003) Bruker Analytical X-ray Systems, Inc., Madison
Sheldrick G (2008) Acta Crystallogr Sect A 64(1):112–122
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Crystallogr 42(2):339–341
McKinnon JJ, Spackman MA, Mitchell AS (2004) Acta Crystallogr Sec t B 60(6):627–668
Wells PR (2007) Group electronegativities. Progress in physical organic chemistry. Wiley, New York, pp 111–145
Seth SK, Sarkar D, Kar T (2011) CrystEngComm 13(14):4528–4535
Batsanov AS, Howard JAK, Albesa-Jové D, Collings JC, Liu Z, Mkhalid IAI, Thibault M-H, Marder TB (2012) Cryst Growth Des 12(6):2794–2802
Ling I, Alias Y, Sobolev AN, Raston CL (2010) CrystEngComm 12(12):4321–4327
Acknowledgments
This research was supported by the National Science Foundation CHE-1305136.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salpage, S.R., Smith, M.D. & Shimizu, L.S. Crystal Structures and Hirshfeld Surface Analyses of 6-Substituted Chromones. J Chem Crystallogr 46, 170–180 (2016). https://doi.org/10.1007/s10870-016-0642-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-016-0642-2