Abstract
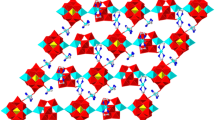
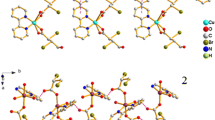
A new bi-supported Dawson-like heteropolytungstate [Cu(phen)(H2O)]2[Cu(phen)(H2O)3]2H[SbW18O60]·5H2O (1) (phen = 1,10-phenanthroline) was synthesized and characterized. The crystal data are listed as follows: C48H59Cu4N8O73SbW18 (Mr = 5600.23), Triclinic, space group P-1, a = 12.4169(9) Å, b = 13.9072(10) Å, c = 14.5712(11) Å, α = 93.8210(10)°, β = 100.0840(10)°, γ = 93.3720(10)°, Z = 1. The counter cation Cu(II) centers show two kinds of coordination environments: octahedral Cu(1) and tetragonal pyramidal Cu(2). The corner-shared Cu(1) and Cu(2) polyhedra are anchored to the surface of polyanion through Cu(1)–O interaction. There are extensive and effective intermolecular hydrogen bonding and π···π interactions to stabilize the structure of compound 1.
Graphical Abstract
A bi-supported Dawson-like heteropolytungstate [Cu(phen)(H2O)]2[Cu(phen)(H2O)3]2H[SbW18O60]·5H2O was synthesized and characterized.







Similar content being viewed by others
References
Miras HN, Cooper GJT, Long DL, Bögge H, Müller A, Streb C, Cronin L (2010) Science 327:72–74
Mishra PP, Pigga J, Liu TB (2008) J Am Chem Soc 130:1548–1549
Yin QS, Tan JM, Besson C, Geletii YV, Musaev DG, Kuznetsov AE, Luo Z, Hardcastle KI, Hill CL (2010) Science 328:342–345
Pradeep CP, Long DL, Newton GN, Song YF, Cronin L (2008) Angew Chem Int Ed 47:4388–4391
Gao PS, Cheng TC, Mak W (2009) J Am Chem Soc 131:18257–18259
Peng ZH (2004) Angew Chem Int Ed Engl 43:930–935
Zheng ST, Zhang J, Yang GY (2008) Angew Chem Int Ed Engl 47:3909–3913
Han ZG, Chai T, Zhai XL, Wang JY, Hu CW (2009) Solid State Sci 11:1998–2002
Song YF, Long DL, Cronin L (2007) Angew Chem Int Ed 46:3900–3904
Coronado E, Galán-Mascarós JR, Giménez-Saiz C, Gómez-García CJ, Martínez-Ferrero E, Almeida M, Lopes EB, Capelli SC, Llusar RM (2004) J Mater Chem 14:1867–1872
Coronado E, Giménez-Saiz C, Gómez-García CJ, Capelli SC (2004) Angew Chem Int Ed 43:3022–3025
Long DL, Burkholder E, Cronin L (2007) Chem Soc Rev 36:105–121
Liu TB, Diemann E, Li HL, Dress AWM, Müller A (2003) Nature 426:59–61
Long DL, Cronin L (2006) Chem Eur J 12:3698–3706
Song YF, McMillan N, Long DL, Thiel J, Ding YL, Chen HS, Gadegaard N, Cronin L (2008) Chem Eur J 14:2349–2354
Pradeep CP, Long DL, Newton GN, Song YF, Cronin L (2008) Angew Chem Int Ed 47:4388–4391
Krebs B, Klein R, Pope MT, Müller A (eds) (1994) Polyoxometalates: from platonic solids to anti-retroviral activity. Kluwer Academic, Dordrecht, p 55
Brown ID, Alternatt D (1985) Acta Cryst B 41:244–247
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (20701011), and the Natural Science Foundation of Hebei province (No. B2011205035), and the Education Department Foundation of Hebei province (Z2006436).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Z., Hao, Q., Wang, Z. et al. Synthesis and Characterization of a New Bi-Supported Dawson-Like Heteropolytungstate. J Chem Crystallogr 43, 1–5 (2013). https://doi.org/10.1007/s10870-012-0376-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-012-0376-8