Abstract
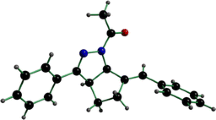
Hydrazine bridged imidazole (1) and its fumarate salt (2) have been synthesized and structurally characterized. Hydrazine bridged imidazole (1) crystallizes in the monoclinic, space group P2(1)/n, with a = 12.809(7) Å, b = 5.102(3) Å, c = 14.401(8) Å, β = 96.859(10)°, V = 934.3(9) Å3, Z = 2. In 1, the two imidazolyl rings are located anti-parallely above and below the benzene planes, respectively. This arrangement leads the imidazolyl rings to be in the trans conformation. In addition at each C=N bond, the larger groups linked to C and N atoms are located in the same side of the double bond, thus in the solid state the conformation of the bis-imidazole (1) is E, E.
Compound 2 crystallizes in the monoclinic, space group P2(1)/n, with a = 14.272(6) Å, b = 4.815(2) Å, c = 17.816(7) Å, β = 92.329(8)°, V = 1223.4(9) Å3, Z = 2. For compound 2, one dimensional chain structure is formed through hydrogen bonds of N–H+···O−. The chains extend in two crossed directions. These adjacent 1D parallel chains form 2D network structure under the interchain CH2–π associations. There also exist interchain CH2···O interactions between crossed chains. Under these weak interactions, 2 displays 3D network structure.
Graphical Abstract
Due to the weak interactions, the complex displays 3D network structure.





Similar content being viewed by others
References
Sundberg RJ, Yilmaz I, Mente DC (1977) Inorg Chem 16:1470
Santoro SW, Joyce GF, Sakthivel K, Gramatikova S, Barbas CF (2000) J Am Chem Soc 122:2433
Arrowsmith J, Jennings SA, Clark AS, Stevens MFG (2002) J Med Chem 45:5458
Betti L, Botta M, Corelli F, Floridi M, Giannaccini ML, Manetti F, Strappaghetti G, Tafi A, Corsano S (2002) J Med Chem 45:3603
Cole C, Reigan P, Gbaj A, Edwards PN, Douglas KT, Stratford IJ, Freeman S, Jaffar M (2003) J Med Chem 46:207
Hay MP, Anderson RF, Ferry DM, Wilson WR, Denny WA (2003) J Med Chem 46:5533
Herrmann WA, Köcher C (1997) Angew Chem Int Ed Engl 36:2162
Bourissou D, Guerret O, Gabbai FP, Bertrand G (2000) Chem Rev 100:39
Jin SW, Chen WZ (2007) Polyhedron 26:3074
Jin SW, Chen WZ (2007) Inorg Chim Acta 12:3756
Jin SW, Wang DQ, Chen WZ (2007) Inorg Chem Comm 10:685
Wu LP, Yamagiwa Y, Kuroda-sowa T, Kamikawa T, Munakata M (1997) Inorg Chim Acta 256:155
Duncan PCM, Goodgame DML, Menzer S, Williams DJW (1996) Chem Commun 2127
Ballester L, Baxter I, Duncan PCM, Goodgame DML, Grachvogel DA, Williams DJ (1998) Polyhedron 17:3613
Ma JF, Yang J, Zheng GL, Li L, Zhang YM, Li FF, Liu JF (2004) Polyhedron 23:553
Ma JF, Yang J, Zheng GL, Li L, Liu JF (2003) Inorg Chem 42:7531
Lennon IC, Ramsden JA (2005) Org Process Res Dev 9:110
Blessing RH (1995) Acta Crystallogr A51:33
Sheldrick GM (1996) SADABS “siemens area detector absorption correction”. University of Göttingen, Göttingen
SHELXTL-PC, version 5.03; Siemens analytical instruments, Madison
Lynch DE, Thomas LC, Smith G, Byriel KA, Kennard CHL (1998) Aust J Chem 51:867
Smith G, White JM (2001) Aust J Chem 54:97
Williams DH, Fleming I (1995) Spectroscopic methods in organic chemistry, 5th edn. McGraw-hill, London
Thirumurugan R, Shanmuga SRS, Shanmugam G, Fun HK, Marappan M, Kandaswamy M (1998) Acta Crystallogr C54:644
Bondi A (1964) J Phys Chem 68:441
Felloni M, Blake AJ, Hubberstey P, Wilson C, Schröder M (2002) CrystEngComm 4:483
Acknowledgments
The authors thank the Zhejiang A & F University Science Foundation for financial support (project No. 2009FK63).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, S., Wang, D. Synthesis and Structural Characterization of Hydrazine Bridged Imidazole and its Fumarate Salt. J Chem Crystallogr 42, 524–528 (2012). https://doi.org/10.1007/s10870-011-9999-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-011-9999-4