Abstract
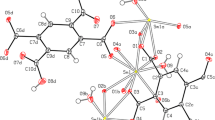
A novel samarium(III) polymer, [Sm2(C6H5COO)6(CH3OH)3]·CH3OH (1) has been synthesized and characterized by single crystal X-ray diffraction and magnetic measurements. Polymer 1 crystallizes in monoclinic space group P2(1)/c, with a = 15.1247(6), b = 18.3351(7), c = 21.1266(6) Å, β = 126.830(2)°, V = 4689.4(3) Å3, and Z = 4. Single crystal X-ray analysis reveals a chain-like structure of 1 consisted of alternating eight- and nine-coordinate Sm(III) ions bridged by benzoate ligands in different coordination modes. A treatment of the variable-temperature magnetic susceptibility using an expression deduced from free-ion approximation and molecular field theory suggests the existence of a weak antiferromagnetic coupling between the samarium ions.
Graphical Abstract
A novel one-dimensional samarium polymer containing alternating eight- and nine-coordinate Sm(III) ions has been generated by incorporating benzoic acid in three different binding modes in its deprotonated state.




Similar content being viewed by others
References
Chen C, Zhang SY, Song HB, Shi W, Zhao B, Cheng P (2009) Inorg Chim Acta 362:2749
Dong YB, Wang P, Ma JP, Zhao XX, Wang HY, Tang B, Huang RQ (2007) J Am Chem Soc 129:4872
Li X, Wu XS, Sun HL, Xu LJ, Zi GF (2009) Inorg Chim Acta 362:2837
Li Y, Zheng FK, Liu X, Zou WQ, Guo GC, Lu CZ, Huang JS (2006) Inorg Chem 45:6308
Lin PH, Burchell TJ, Ungur L, Chibotaru LF, Wernsdorfer W, Murugesu M (2009) Angew Chem Int Ed 48:9489
Yan PF, Zhang FM, Li GM, Zhang JW, Sun WB, Suda M, Einaga Y (2009) J Solid State Chem 182:1685
Che TL, Gao QC, Zhang WP, Nan ZX, Li HX, Cai YG, Zhao JS (2009) Koord Khim 35:723
Zheng SL, Yang JH, Yu XL, Chen XM, Wong WT (2004) Inorg Chem 43:830
Chen ZL, Jiang CF, Yan WH, Liang FP, Batten SR (2009) Inorg Chem 48:4674
Wang XL, Qin C, Wang EB, Su ZM (2006) Chem Eur J 12:2680
Rueff JM, Masciocchi N, Rabu P, Sironi A, Skoulios A (2001) Eur J Inorg Chem 2001:2843
Zhou Y, Yuan D, Jiang F, Xu Y, Hong M (2006) J Mol Struct 796:203
Ren N, Zhang JJ, Xu SL, Wang RF, Wang SP (2005) Thermochim Acta 438:172
Contaldi S, Di Nicola C, Garau F, Karabach YY, Martins LMDRS, Monari M, Pandolfo L, Pettinari C, Pombeiro AJL (2009) Dalton Trans 4928
Khanra S, Weyhermüller T, Bill E, Chaudhuri P (2006) Inorg Chem 45:5911
Taylor MD, Carter CP, Wynter CI (1968) J Inorg Nucl Chem 30:1503
Sheldrick GM (1997) SHELXS-97, program for crystal structure solution. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97, program for crystal structure refinement. University of Göttingen, Germany
Singh UP, Kumar R, Upreti S (2007) J Mol Struct 831:97
Lu ZD, Wen LL, Yao J, Zhu HZ, Meng QJ (2006) CrystEngComm 8:847
Andruh M, Bakalbassis E, Kahn O, Trombe JC, Porcher P (1993) Inorg Chem 32:1616
Wang Y, Li XL, Wang TW, Song Y, You XZ (2010) Inorg Chem 49:969
Acknowledgments
We thank the Changchun Institute of Applied Chemistry (Start-up) and the National Natural Science Foundation of China (Grants 20871113 and 20921002) for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lin, SY., Zhao, L., Xu, GF. et al. Synthesis, Crystal Structure and Magnetic Property of a One-Dimensional Samarium(III) Coordination Polymer. J Chem Crystallogr 41, 77–81 (2011). https://doi.org/10.1007/s10870-010-9842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9842-3