Abstract
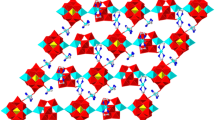
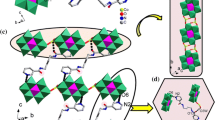
Two new metal complexes supported by {VO3}n n− chains, [M(dpa)V2O6] (1, M = Zn2+; 2·H2O, M = Cu2+; dpa = 2,2′-dipyridylamine), have been synthesized hydrothermally and characterized by elemental analysis, TG analysis, IR spectroscopy and single-crystal X-ray diffraction. Crystal data: [Zn(dpa)V2O6] 1, Triclinic, P-1, a = 9.663(7) Å, b = 10.617(7) Å, c = 15.114(10) Å, α = 105.678(10)°, β = 104.772(9)°, γ = 94.021(10)°, Z = 2; [Cu(dpa)V2O6]·H2O 2·H2O, Monoclinic, C2, a = 20.543(3) Å, b = 7.2460(9) Å, c = 10.4853(13) Å, β = 111.318(2)°, Z = 4. Complex 1 is constructed from sinusoidal {VO3}n n− chains with {Zn(dpa)}2+ fragments spanning the adjacent troughs and crests into an 1D ribbon-like structure. Complex 2 is built up by linking {VO3}n n− chains via pairs of symmetrical {Cu(dpa)}2+ fragments into a 2D layered structure. The Zn(II) and Cu(II) ions exhibit tetrahedral and square pyramidal coordination environments, respectively. The formation of the two isomers is attributed to the flexibility of {VO3}n n− chains and the different coordination configurations of the two metal ions. There exist significant π–π stacking and hydrogen bonding interactions in complexes 1 and 2.
Index Abstract
Single crystal X-ray diffraction analysis reveals that two new metal complexes, [M(dpa)V2O6] (1, M = Zn2+; 2·H2O, M = Cu2+; dpa = 2,2′-dipyridylamine), are constructed from {VO3}n n− chains and {M(dpa)}2+ fragments into 1D and 2D structures.





Similar content being viewed by others
References
Hagrmann PJ, Hagrmann D, Zubieta J (1999) Angew Chem Int Ed 38:2638
Centi G, Trifro F, Ebbner JR, Franchetti VM (1988) Chem Rev 88:55
Zheng LM, Zhao JS, Li KH, Zhang LY, Liu Y, Xin XQ (1999) J Chem Soc Dalton Trans 939
DeBord JRD, Zhang Y, Aushalter RC, Zubieta J, O’Connor CJ (1996) J Solid State Chem 122:251
Finn RC, Sims J, O’Connor CJ (2002) J Chem Soc Dalton Trans 159
Liu CM, Gao S, Hu HM, Jin XL, Kou HZ (2002) J Chem Soc Dalton Trans 598
LaDuca RL, Rarig RS, Zubieta J (2001) Inorg Chem 40:607
LaDuca RL, Finn R, Zubieta J (1999) Chem Commun 1669
Sun CY, Wang EB, Xiao DR, An HY, Xu L (2007) J Mol Struct 840:53
Xiao DR, Wang ST, Hou Y, Wang EB, Li YG, An HY, Xu L, Hu CW (2004) J Mol Struct 692:107
Devi RN, Zubieta J (2002) Inorg Chim Acta 338:165
Lin BZ, Pei XK, Liu PD (2003) Inorg Chem Commun 6:1362
Hagrman PJ, Zubieta J (2001) Inorg Chem 40:2800
Zheng LM, Wang XQ, Jacobson AJ (2001) J Mater Chem 11:1100
Hagrman PJ, Bridges C, Greedan E, Zubieta J (1999) J Chem Soc Dalton Trans 2901
Lin BZ, Liu SX (2000) Polyhedron 19:2521
Sheldrick GM (1997) SHELX 97, program for crystal structure refinement. University of Göttingen, Germany
Acknowledgment
This work was financially supported by the National Natural Science foundation of China (Grant No. 20773057) and Liaoning Provincial Educational Commission (Project No. 605L207).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, Th., Zhang, X., Wang, Q. et al. Two Metal Complexes Supported by {VO3}n n− Chains: Hydrothermal Syntheses and Crystal Structures of [M(dpa)V2O6] (M = Zn(II) and Cu(II); dpa = 2,2′-dipyridylamine). J Chem Crystallogr 41, 64–68 (2011). https://doi.org/10.1007/s10870-010-9838-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9838-z