Abstract
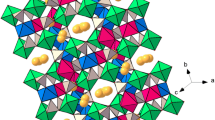
Single crystals of iron and manganese phosphate Fe6.36Mn0.64(PO3(OH))4(PO4)2 was synthesized by hydrothermal method. The compound crystallizes in the Fe7(PO4)6 structure type and is isotypic with the solid solution \( {\text{M}}_{{7 - {\text{x}}}} {\text{M}}_{\text{x}}^{\prime} \left( {{\text{HPO}}_{4} } \right)_{4} \left( {{\text{PO}}_{4} } \right)_{2} \) where M is Fe, Co, Mg, Mn. The compound is triclinic, P-1, a = 6.571(5), b = 7.993(3), c = 9.547(2) Ǻ, α = 103.97(1)°, β = 109.29(2)°, γ = 101.57(3)°. The structure is based on a three-dimensional framework of distorted edge-sharing MO6 and MO5 polyhedra, forming infinite chains, which are interlinked by corner-sharing with PO4 tetrahedra. The formula unit is centrosymmetric, with all atoms in general positions except for one Fe atom, which has site symmetry −1.
Graphical Abstract:



Similar content being viewed by others
References
Atkins WC (1974) US Patent 3855279 to Eastman Kodak Co
Ai M, Ohdan K (1997) Appl Catal 150:13
Ai M, Ohdan K (1997) Appl Catal 165:461
Ai M, Ohdan K (1997) Bull Chem Soc Jpn 70:1995
Ai M, Ohdan K (1997) Stud Surf Sci Catal 110:527
Boudraa M, Merazig H, Bouacida S, Benard-Rocherulle P, Roisnel T (2007) Acta Crystallogr E63:i168–i169
Lightfoot P, Cheetham AK (1988) Acta Crystallogr C44:1331–1334
Kolitsh U, Bartu P (2004) Acta Crystallogr C60:i94
North ACT, Phillips DC, Mathews FS (1968) Acta Crystallogr Sect A
Sheldrick GM (2008) Acta Crystallogr A 64:112–122
Moore PB, Araki T (1973) Am Mineral 58:302–307
Menchetti S, Sabelli C (1973) Acta Crystallogr B29:2541
Larrea ES, Mesa JL, Pizarro JL, Arriortua MI, Rojo T (2007) J Solid State Chem 180:1686–1697
Moore PB, Araki T (1975) Am Mineral 60:454–459
Calvo C (1968) Am Mineral 53:742–750
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belfguira, N., Walha, S., Ben Salah, A. et al. Hydrothermal Synthesis and Structure of Fe6.36Mn0.64(PO3(OH))4(PO4)2 . J Chem Crystallogr 40, 1125–1128 (2010). https://doi.org/10.1007/s10870-010-9807-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9807-6