Abstract
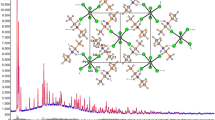
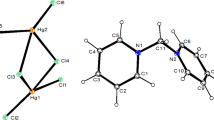
The synthesis and Crystal structure are given for the bis (N,N,N′,N′-tetramethylethylendiammonium) octaiodo pentachloroantimonate (III) salt. An X-ray investigation has shown that the title compound crystallizes in a monoclinic system, space group P21/m with the following lattice parameters a = 9.786(2) Ǻ, b = 14.024(5) Ǻ, c = 14.336(3) Ǻ, β = 91.35(2)° and Z = 2. The structure was solved from 3085 independent reflections with R 1 = 0.0402 and wR 2 = 0.0952 and refined with 152 parameters. The structure shows a layer arrangement perpendicular to the \( \vec{b} \)-axis: planes of [SbCl5]2−, I3 − and I5 − anions alternate with planes of [(CH2)2(NH(CH3)2)2]2+ cations. The [SbCl5]2− square pyramids present an active lone electron pair (LEP) on the Sb atoms and they are interconnected by means of N–H···Cl hydrogen bond originating from [(CH2)2(NH(CH3)2)2]2+ entities. The polyiodides (I3 − and I5 −) anions form a planer zigzag polymeric chains via I···I linking interactions. These chains are linked to the [SbCl5]2− anions by a long range contact.
Index Abstract
Built for [(CH2)2(NH(CH3)2)2]2+ entities, [SbCl5]2−, I3 − and I5 − anions.



Similar content being viewed by others
References
Bujak M, Zaleski J (2003) J Mol Struct 647:121
Kulicka B, Jakubas R, Ciunika Z (2004) J Phys Chem Solids 65:871
Bujak M, Zaleski J (1999) Acta Crystallogr C55:1775
Tarasiewicz J, Jakubas R, Baran J, Pietraszko A (2004) J Mol Struct 697:161
Zdanowska-Fraczek M, Jakubas R, Krupski M (2004) J Phys Chem Solids 65:1679
Piecha A, Kinzhybalo V, Slepokura K, Jakubas R (2007) J Solid State Chem 180:265
Chaabouni S, Kamoun S, Daoud A, Jouini T (1997) J Chem Crystallogr 27:401
Chaabouni S, Kamoun S, Jaud J (1998) Mater Res Bull 33:377
Svenson PH, Kloo L (2003) Chem Rev 103:1649
Blake AJ, Devillanova FA, Gould RO, Li WS, Lippolis V, Parsons S, Radek C, Schröder M (1998) Chem Soc Rev 27:195
Horn CJ, Blake AJ, Champness NR, Lippolis V, Schröder M (2003) J Chem Soc Chem Commun 1488
Charlot G (1974) Chimie analytique quantitative, vol II. Masson and Cie, Paris
CAD-4 Express Software Enraf-Nonius (1994) Delft, The Netherlands
Sheldrick GM (1977) SHELXS-97. Program for crystal structure solution. University of Göttingen, Germany
Sheldrick GM (1977) SHELXL-97. Program for crystal structure refinement. University of Göttingen, Germany
Farrugia LJ (1999) J Appl Crystallogr 32:837
International Tables for X-Ray Crystallogr (1992) vol C. Kluwer, Dordrecht
Vogler A, Kunkely H (1993) In: Kalyanasundaram K, Grätzel M (eds) Photosensitation and photocatalysis using inorganic and organometallic compounds. Kluwer, Dordrecht, p 71
Shannon RD (1976) Acta Crystallogr A32:751
Bauer WH (1974) Acta Crystallogr B30:1195
Brown ID, Altermatt D (1985) Acta Crystallogr B41:244
Brown ID, Wu KK (1976) Acta Crystallogr B32:1957
Karle IL (1955) J Chem Phys 23:1739
Van Bolhuis F, Koster PB, Migehelsen T (1967) Acta Crystallogr 23:90
Nyburg SC, Faerman CH (1985) Acta Crystallogr B41:274
Gharbi A, Charfi M, Jouini A (1996) Acta Crystallogr C52:2246
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kharrat, H., Kamoun, S., Ayedi, H.F. et al. Synthesis and Crystal Structure of Bis (N,N,N′,N′-tetramethylethylendiammonium) Octaiodo Pentachloroantimonate (III). J Chem Crystallogr 40, 721–725 (2010). https://doi.org/10.1007/s10870-010-9725-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9725-7