Abstract
A new acetyl phosphorylamidate P(O)[NHC(O)C6H4(4-NO2)][N(CH(CH3)2)(CH2C6H5)]2 has been synthesized and characterized by elemental analysis, 1H, 13C and 31P NMR, IR and single crystal X-ray diffraction. Single crystal X-ray analysis shows that it belongs to triclinic system, space group \( P\bar{1} \), with a = 10.5868(16) Å, b = 11.8058(18) Å, c = 12.4364(19) Å, α = 65.410(3)°, β = 67.492(4)°, γ = 85.879(3)°, V = 1,298.6(3) Å3, and Z = 2. The intermolecular PO···HN hydrogen bond makes H-bonded dimer of molecule with Ci symmetry. In the crystal network, the dimers are aggregated in the chain arrays through π-stacking between p-NO2–C6H4–C(O)–NH– moieties. Moreover, weak C–H···O and C–H···π interactions exist in the crystal network.
Graphical Abstract
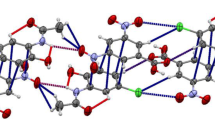
In the crystal network of P(O)[NHC(O)C6H4(4-NO2)][N(CH(CH3)2)(CH2C6H5)]2, the H-bonded dimers with Ci symmetry are aggregated in the chain arrays through π-stacking between p-NO2–C6H4–C(O)–NH– moieties.







Similar content being viewed by others
References
Gubina KE, Amirkhanov VM (2000) Z Naturforsch 55b:1015–1019
Barak D, Ordentlich A, Kaplan D, Barak R, Mizrahi D, Kronman C, Segall Y, Velan B, Shafferman A (2000) Biochemistry 39:1156–1161. doi:10.1021/bi992009n
Mallender WD, Szegletes T, Rosenberry TL (2000) Biochemistry 39:7753–7763. doi:10.1021/bi000210o
Jaroslav K, Swerdloff F (1985) US Patent 4, 517, 003
Pourayoubi M, Sabbaghi F (2007) Acta Crystallogr E63:o4366
Yazdanbakhsh M, Sabbaghi F (2007) Acta Crystallogr E63:o4318
Bruker S (1998) Bruker molecular analysis research tool, v. 5.059. Bruker AXS, Madison
Sheldrick GM (1998) SHELXTL v. 5.10. Structure determination software suit. Bruker AXS, Madison
Sheldrick GM (1998) SADABS v. 2.01. Bruker/Siemens area detector absorption correction program. Bruker AXS, Madison
Corbridge DEC (1995) Phosphorus, an outline of its chemistry, biochemistry and technology, 5th edn. Elsevier, The Netherlands
Gholivand K, Pourayoubi M (2004) Z Anorg Allg Chem 630:1330–1335. doi:10.1002/zaac.200400136
Gholivand K, Pourayoubi M, Shariatinia Z, Mostaanzadeh H (2005) Polyhedron 24:655–662. doi:10.1016/j.poly.2005.01.010
Gholivand K, Shariatinia Z, Pourayoubi M (2005) Z Anorg Allg Chem 631:961–967. doi:10.1002/zaac.200400517
Gholivand K, Shariatinia Z, Pourayoubi M (2006) Polyhedron 25:711–721. doi:10.1016/j.poly.2005.07.035
Gholivand K, Pourayoubi M, Mostaanzadeh H (2004) Anal Sci 20:x51–x52. doi:10.2116/analscix.20.x51
Gholivand K, Shariatinia Z, Pourayoubi M (2006) Z Anorg Allg Chem 632:160–166. doi:10.1002/zaac.200500326
Gholivand K, Hosseini Z, Pourayoubi M, Shariatinia Z (2005) Z Anorg Allg Chem 631:3074–3079. doi:10.1002/zaac.200500274
Gholivand K, Shariatinia Z, Pourayoubi M (2005) Z Naturforsch 60:67–74
Pourayoubi M, Ghadimi S, Ebrahimi Valmoozi AA (2007) Acta Crystallogr E63:o4093
Ghadimi S, Pourayoubi M, Ebrahimi Valmoozi AA (2009) Z Naturforsch 64b:565–569
Calhorda MJ (2000) Chem Commun (Camb) 10:801–809. doi:10.1039/a900221i (and references cited therein)
Steiner T (1998) N J Chem 22:1099–1103. doi:10.1039/a804121k
Gholivand K, Pourayoubi M (2004) Z Kristallogr NCS 219:314–316. doi:10.1524/zkri.219.6.314.34639
Li X-Z, Yang G-M, Liao D-Z, Jiang Z-H, Yan S-P (2003) J Chem Crystallogr 33:5–9. doi:10.1023/A:1021339530884
Gholivand K, Shariatinia Z, Pourayoubi M (2005) Z Kristallogr NCS 220:65–66
Malone JF, Murray CM, Charlton MH, Docherty R, Lavery AJ (1997) J Chem Soc, Faraday Trans 93:3429–3436. doi:10.1039/a700669a
Wipff G, Boehme C (2002) Inorg Chem 41:727–737. doi:10.1021/ic010658t
Iriarte AG, Erben MF, Gholivand K, Jios JL, Ulic SE, Della Védova CO (2008) J Mol Struct 886:66–71. doi:10.1016/j.molstruc.2007.10.036
Narula PM, Day CS, Powers BA, Odian MA, Lachgar A, Pennington WT, Noftle RE (1999) Polyhedron 18:1751–1759. doi:10.1016/S0277-5387(99)00055-8
Acknowledgments
Support of this investigation by Islamic Azad University-Zanjan Branch is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pourayoubi, M., Sabbaghi, F. Synthesis, Spectroscopic Characterization and Crystal Structure of a New Acetyl phosphorylamidate P(O)[NHC(O)C6H4(4-NO2)][N(CH(CH3)2)(CH2C6H5)]2 . J Chem Crystallogr 39, 874–880 (2009). https://doi.org/10.1007/s10870-009-9582-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9582-4