Abstract
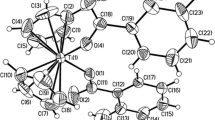
Based on the reaction between Cp2TiCl2 and substituted salicylic acids in the presence of β-cyclodextrin polymer (β-CDP), three four-coordinated titanocene complexes [(η5-C5H5)2Ti(S,O′)(OCC6H4)·(C6H6)0.5] (1), [(η5-C5H5)2Ti{(O,O′)(3,5-Cl2-OCC6H2)}] (2) and [(η5-C5H5)2Ti{(O,O′)(3,5-(NO2)2-OCC6H2)}] (3) were synthesized in high yields and their crystal structures have been determined by single-crystal X-ray diffraction. The structure of 1 has a Monoclinic space group P21/c with a = 8.313(3) Å, b = 9.960(4) Å, c = 22.330(8) Å, β = 111.856(11)° and Z = 4. The structure of 2 has a Monoclinic space group P21/c with a = 8.0577(13) Å, b = 8.9022(14) Å, c = 21.977(4) Å, β = 96.298(3)° and Z = 4. The structure of 3 has a Triclinic space group P-1 with a = 8.1687(11) Å, b = 8.3027(11) Å, c = 12.7164(17) Å, α = 102.930(2)°, β = 100.479(2)°, γ = 95.458(2)° and Z = 2. Each of the complexes exhibits a three-dimensional framework constructed through weak interactions, which are hydrogen bonding, π–π stacking and C–H···π interactions. It was found that the variation of the substituted salicylate ligands affect the weak interactions as well as the specific framework structure that forms.
Graphical Abstract
Three four-coordinated titanocene complexes [(η5-C5H5)2Ti(S,O′)(OCC6H4)·(C6H6)0.5] (1), [(η5-C5H5)2Ti{(O,O′)(3,5-Cl2-OCC6H2)}] (2) and [(η5-C5H5)2Ti{(O,O′)(3,5-(NO2)2-OCC6H2)}] (3) were synthesized in high yields, each of the complexes exhibits a three-dimensional framework constructed through weak interactions. It was found that simple variation of the substituted salicylate ligands affect the weak interactions as well as the specific framework structure that forms.

.











Similar content being viewed by others
References
Yaghi OM, Li H, Groy TL (1997) Inorg Chem 36:4292. doi:10.1021/ic970423a
Cui Y, Ngo HL, Lin WB (2002) Inorg Chem 41:1033. doi:10.1021/ic015627c
Kil SM, Myunghyun PS (2000) J Am Chem Soc 122:6834. doi:10.1021/ja000642m
Fujita M, Kwon YJ, Sasaki O, Yamaguchi K, Ogura K (1995) J Am Chem Soc 117:7287. doi:10.1021/ja00132a046
Bettencourt-Dias A (2005) Inorg Chem 44:2734. doi:10.1021/ic048499b
Goodgame DML, Grachvogel DA, Willams DJ (2002) J Chem Soc, Dalton Trans 2259. doi:10.1039/b202938n
Du M, Bu XH, Guo YM, Liu H (2002) Inorg Chem 41:4904. doi:10.1021/ic025559+
Zaworotko MJ, Moulton B (2001) Chem Rev 101:1629. doi:10.1021/cr9900432
Eddaoudi M, Moler DB, Li H, Chen B, Reineke TM, Keeffe MO, Yaghi OM (2001) Acc Chem Res 34:319. doi:10.1021/ar000034b
MacDonald JC, Whitesides GM (1994) Chem Rev 94:2383. doi:10.1021/cr00032a007
Guckian KM, Schweitzer BA, Ren RXF, Sheils CJ, Tahmassebi DC, Kool ET (2000) J Am Chem Soc 122:2213. doi:10.1021/ja9934854
Chen CL, Su CY, Cai YP, Zhang HX, Xu AW, Kang BS, Loye HC (2003) Inorg Chem 42:3738. doi:10.1021/ic0341035
Nishio M (2004) Cryst Eng Comm 6:130. doi:10.1039/b313104a
Hunks WJ, Jennings MC, Puddephatt RJ (2002) Inorg Chem 41:4590. doi:10.1021/ic020178h
Ko JW, Min KS, Suh MP (2002) Inorg Chem 41:2151. doi:10.1021/ic011281u
Noveron JC, Lah MS, Sesto RED, Arif AM, Miller JS, Stang PJ (2002) J Am Chem Soc 124:6613. doi:10.1021/ja0200241
Burlakov VV, Troyanov SI, Letov AV, Strunkina LI, Minacheva MK, Furin GG, Rosenthal U, Shur VB (2000) J Organomet Chem 598:243. doi:10.1016/S0022-328X(99)00716-0
Bryliakov KP, Babushkin DE, Talsi EP, Voskoboynikov AZ, Gritzo H, Schrőder L, Damrau HRH, Wieser U, Schaper F, Brintzinger HH (2005) Organometallics 24:894. doi:10.1021/om0492496
Borrelli M, Busico V, Cipullo R, Ronca S (2002) Macromolecules 35:2835. doi:10.1021/ma011557h
Takahashi T, Bao FY, Gao GH, Ogasawara M (2003) Org Lett 5:3479. doi:10.1021/ol035277t
Halterman RL (1992) Chem Rev 92:965. doi:10.1021/cr00013a011
Beckhaus R, Sang J, Wagner T, Ganter B (1996) Organometallics 15:1176. doi:10.1021/om950783a
Halterman RL, Chen ZL, Khan MA (1996) Organometallics 15:3957. doi:10.1021/om960271b
Harrod JF (2000) Coord Chem Rev 206:493. doi:10.1016/S0010-8545(00)00335-0
Mokdsi G, Harding MM (1998) J Organomet Chem 565:29. doi:10.1016/S0022-328X(98)00441-0
Kuo LY, Kanatzidis MG, Sabat M, Tipton AL, Tobin JM (1991) J Am Chem Soc 113:9027. doi:10.1021/ja00024a002
Harding MM, Mokdsi G, Mackay JP, Prodigalidad M, Lucas SW (1998) Inorg Chem 37:2432. doi:10.1021/ic971205k
Meléndez E (2002) Crit Rev Oncol Hematol 42:309. doi:10.1016/S1040-8428(01)00224-4
Anderberg PI, Harding MM, Luck IJ, Turner P (2002) Inorg Chem 41:1365. doi:10.1021/ic010876m
Causey PW, Baird MC (2004) Organometallics 23:4486. doi:10.1021/om049679w
Radim B, Ivana C, Martin P, Ivan P (2004) Appl Organomet Chem 18:262. doi:10.1002/aoc.623
Song LC, Han C, Hu QM, Zhang ZP (2004) Inorg Chim Acta 357:2199. doi:10.1016/j.ica.2003.06.011
Guo M, Sun H, Bihari S, Parkinson JA, Gould RO, Parsons S, Sadler PJ (2000) Inorg Chem 39:206. doi:10.1021/ic990669a
Liu FC, Chen KY, Chen JH (2003) Inorg Chem 42:1758. doi:10.1021/ic020597e
Kirchbauer FG, Pellny PM, Sun H, Burlakov VV, Arndt P, Baumann W, Spannenberg A, Rosenthal U (2001) Organometallics 20:5289. doi:10.1021/om010404f
Niehues M, Erker G, Kehr G, Schwab P, Frőhlich R (2002) Organometallics 21:2905. doi:10.1021/om020088k
Wada K, Itayama N, Watanabe N, Bundo M, Kondo T, Mitsudo T (2004) Organometallics 23:5824. doi:10.1021/om040082q
Thewalt U, Schinnerling P (1991) J Organomet Chem 418:191. doi:10.1016/0022-328X(91)86366-X
Klein H, Doppert K, Thewalt U (1985) J Organomet Chem 280:203. doi:10.1016/0022-328X(85)88093-1
Koepf H, Grabowski S, Voigtlaender R (1981) J Organomet Chem 216:185. doi:10.1016/S0022-328X(00)85759-9
Klein HP, Thewalt U, Doppert K, Sanchez-Delgado R (1982) J Organomet Chem 236:189. doi:10.1016/S0022-328X(00)87074-6
Guethner T, Thewalt U (1989) J Organomet Chem 371:43. doi:10.1016/0022-328X(89)85206-4
Aucott SM, Kilian P, Milton HL, Robertson SD, Slawin AMZ, Woollins JD (2005) Inorg Chem 44:2710. doi:10.1021/ic048483l
Lu ZR, Gao SQ, Zhou YK, Wu SZ (1994) Chin J Appl Chem 11:28
Edwards DA, Mahon MF, Paget TJ, Summerhill NW (2001) Transition Met Chem (Kyoto) 26:116
Gao Z, Sun P, Gao L, Han L, Yuan W, Liu Y, Zhang J, Lv M (2004) Acta Chim Sin 62:979
Gao Z, Hu D, Gao L, Zhang X, Zhang Z, Liang Q (2001) J Organomet Chem 629:47. doi:10.1016/S0022-328X(01)00832-4
Gao Z (2000) Acta Chim Sin 58:343
Gao Z, Zhao X (2003) Polymer (Guildf) 44:4519. doi:10.1016/S0032-3861(03)00416-6
Sheldrick GM (1997) SHELX-97, program package for crystal structure solution and refinement. University of Göttingen, Germany
Wang J, Zheng C, Maguire JA, Hosmane NS (2003) Organometallics 22:4839. doi:10.1021/om0340802
Xu JW, Wang WL, Lai YH (2005) Tetrahedron 61:9248. doi:10.1016/j.tet.2005.07.071
Kubicki M (2004) J Mol Struct 698:67. doi:10.1016/j.molstruc.2004.04.018
Thallapally PK, Chakraborty K, Carrell HL, Kothab S, Desirajua GR (2000) Tetrahedron 56:6721. doi:10.1016/S0040-4020(00)00493-2
Du M, Guo JH, Zhao XJ (2004) J Mol Struct 701:119. doi:10.1016/j.molstruc.2004.05.027
Boyle PD, Davidson SE, Godfrey SM, Pritchard RG (2001) Inorg Chim Acta 325:211. doi:10.1016/S0020-1693(01)00635-1
Janiak C (2000) J Chem Soc Dalton Trans 3885. doi:10.1039/b003010o
Abrahams BF, Hudson TA, Robson R (2004) J Am Chem Soc 126:8624. doi:10.1021/ja048293+
Burlakov VV, Arndt P, Baumann W, Spannenberg A, Rosenthal U, Letov AV, Lyssenko KA, Korlyukov AA, Strunkina LI, Minacheva MK, Shur VB (2001) Organometallics 20:4072. doi:10.1021/om0103052
Alekseev NV, Ronova IA (1966) Zh Strukt Khim 7:103
Dang Y, Geise HJ, Dommisse R, Esmans E, Desseyn HO (1990) J Organomet Chem 381:333. doi:10.1016/0022-328X(90)80063-6
Chandra K, Sharma AK, Garg BS, Singh RP (1980) J Inorg Nucl Chem 42:187. doi:10.1016/0022-1902(80)80238-7
Rotzinger FP, Kesselman-Truttmann JM, Hug SJ, Shklover V, Graltze M (2004) J Phys Chem B 108:500. doi:10.1021/jp0360974
Acknowledgments
The financial support given by the National Natural Science Foundation of China (20771071), the Program for New Century Excellent Talents in University of China and Natural Science Foundation of Shaanxi Province (2007B06) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Gao, Z., Zhang, C. et al. Synthesis and Structural Characterization of Substituted Salicylate Titanocene Complexes: Three Supramolecular Frameworks Determined by Weak Interactions. J Chem Crystallogr 39, 623–631 (2009). https://doi.org/10.1007/s10870-009-9537-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9537-9