Abstract
Sex hormone-binding globulin (SHBG) is a binding protein that regulates the availability of steroid hormones in the plasma. Although best known as a steroid carrier, recent studies have associated SHBG in modulating behavioral aspects related to sexual receptivity. Among steroids, estradiol (17β-estradiol, oestradiol or E2), documented as the most active endogenous female hormone, exerts important physiological roles in both reproductive and non-reproductive functions. In this framework, we employed molecular dynamics (MD) and docking techniques for quantifying the interaction energy between a complex aqueous solution, composed by different salts, SHBG and E2. As glucose concentration resembles measured levels in diabetes, special emphasis was devoted to analyzing the interaction energy between this carbohydrate, SHBG and E2 molecules. The calculations revealed remarkable interaction energy between glucose and SHBG surface. Surprisingly, a movement of solute components toward SHBG was observed, yielding clusters surrounding the protein. The high energy and short distance between glucose and SHBG suggests a possible scenario in favor of a detainment state between the sugar and the protein. In this context, we found that glucose clustering does not insert modification on binding site area nor over binding energy SHBG-E2 complex, in spite of protein superficial area increment. The calculations also point to a more pronounced interaction between E2 and glucose, considering the hormone immersed in the solution. In summary, our findings contribute to a better comprehension of both SHBG and E2 interplay with aqueous solution components.







Similar content being viewed by others
References
Salis, A., Ninhamb, B.W.: Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 43, 7358–7377 (2014)
Hille, B.: Ion Channels of Excitable Membranes. Sinauer Associates, Sunderland (2001)
Kramer, R.M., Shende, V.R., Motl, N., Pace, C.N., Scholtz, J.M.: Toward a molecular understanding of protein solubility: increased negative surface charge correlates with increased solubility. Biophys. J. 102, 1907–1915 (2012)
Peggion, E., Mammi, S., Palumbo, M., Moroder, L., Wünsch, E.: Interaction of calcium ions with peptide hormones of the gastrin family. Biopolymers 22, 2443–2457 (1983)
Saboury, A.A., Atri, M.S., Sanati, M.H., Moosavi-Movahedi, A.A., Hakimelahi, G.H., Sadeghi, M.: A thermodynamic study on the interaction between magnesium ion and human growth hormone. Biopolymers 81, 120–126 (2006)
Gould, K.G., Ansari, A.H.: Electrolyte interactions in cervical mucus and their relationship to circulating hormone levels. Contraception 23, 507–516 (1981)
van Gunsteren, W.F., Mark, A.E.: On the interpretation of biochemical data by molecular dynamics computer simulation. Eur. J. Biochem. 204, 947–961 (1992)
Santos, E.S., Souza, L.C., Assis, P.N., Almeida, P.F., Ramos, E.S.: Novel potential inhibitors for adenylylsulfate reductase to control souring of water in oil industries. J. Biomol. Struct. Dyn. 32, 1780–1792 (2014)
Santos, S.E., Gritta, D.H.S., Almeida, J.S.: Analysis of interactions between potent inhibitors of ATP sulfurylase via molecular dynamics. Mol. Simul. 42, 605–610 (2015)
Fraternali, F., Van Gunsteren, W.F.: An efficient mean solvation force model for use in molecular dynamics simulations of proteins in aqueous solution. J. Mol. Biol. 256, 939–948 (1996)
Soares, C.M., Teixeira, V.H., Baptista, A.M.: Protein structure and dynamics in nonaqueous solvents: insights from molecular dynamics simulation studies. Biophys. J. 84, 1628–1641 (2003)
Friedman, R.: Ions and the protein surface revisited: extensive molecular dynamics simulations and analysis of protein structures in alkali-chloride solutions. J. Phys. Chem. B 115, 9213–9223 (2011)
Ahlstrom, P., Teleman, O., Jonsson, B., Forsén, S.: Molecular dynamics simulation of parvalbumin in aqueous solution. J. Am. Chem. Soc. 109, 1541–1551 (1987)
Avvakumov, G.V., Muller, Y.A., Hammond, G.L.: Steroid-binding specificity of human sex hormone-binding globulin is influenced by occupancy of a zinc-binding site. J. Biol. Chem. 275, 25920–25925 (2000)
Boström, M., Williams, D.R., Ninham, B.W.: Specific ion effects: why the properties of lysozyme in salt solutions follow a Hofmeister series. Biophys. J. 85, 686–694 (2003)
Stanaway, S.E.R.S., Gill, G.V.: Protein glycosylation in diabetes mellitus: biochemical and clinical considerations. Pract. Diab. Int. 17, 21–25 (2000)
Neelofar, K., Arif, Z., Alam, K., Ahmad, J.: Hyperglycemia-induced structural and functional changes in human serum albumin of diabetic patients: a physico-chemical study. Mol. BioSyst. 12, 2481–2489 (2016)
Roy, A., Sil, R., Chakraborti, A.S.: Non-enzymatic glycation induces structural modifications of myoglobin. Mol. Cell. Biochem. 338, 105–114 (2010)
Anderson, D.C.: Sex-hormone-binding globulin. Clin. Endocrinol. 3, 69–96 (1974)
Damassa, D.A., Cates, J.M.: Sex hormone-binding globulin and male sexual development. Neurosci. Biobehav. Rev. 19, 165–175 (1995)
Selby, C.: Sex hormone binding globulin: origin, function and clinical significance. Ann. Clin. Biochem. 27, 532–541 (1990)
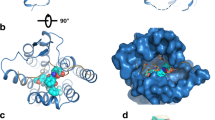
Grishkovskaya, I., Avvakumov, G.V., Sklenar, G., Dales, D., Hammon, G.L., Muller, Y.A.: Crystal structure of human sex hormone-binding globulin: steroid transport by a laminin G-like domain. EMBO J. 19, 504–512 (2000)
Ding, E.L., Song, Y., Manson, J.E., Hunter, D.J., Lee, C.C., Rifai, N., Buring, J.E., Gaziano, J.M., Liu, S.: Sex hormone–binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361, 1152–1163 (2009)
Hautanen, A.: Synthesis and regulation of sex hormone-binding globulin in obesity. Int. J. Obes. Relat. Metab. Disord. Suppl. 2, S64–S70 (2000)
Wang, Y.M., Bayliss, D.A., Millhorn, D.E., Petrusz, P., Joseph, D.R.: The androgen-binding protein gene is expressed in male and female rat brain. Endocrinology 127, 3124–3130 (1990)
Gnanasekar, M., Suleman, F.G., Ramaswamy, K., Caldwell, J.D.: Identification of sex hormone binding globulin-interacting proteins in the brain using phage display screening. Int. J. Mol. Med. 24, 421–426 (2009)
Simpson, E., Santen, R.J.: Celebrating 75 years of oestradiol. J. Mol. Endocrinol. 55, T1–T20 (2015)
Södergård, R., Bäckström, T., Shanbhag, V., Carstensen, H.: Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J. Steroid Biochem. 16, 801–810 (1982)
Anstead, G.M., Carlson, K.E., Katzenellenbogen, J.A.: The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 62, 268–303 (1997)
Toran-Allerand, C.D., Tinnikov, A.A., Singh, J.R., Nethrapalli, I.S.: 17 alpha-estradiol: a brain-active estrogen? Endocrinology 146, 3843–3850 (2005)
Danzo, B.J., Eller, B.C.: The presence of a cytoplasmic estrogen receptor in sexually mature rabbit epididymides: comparison with the estrogen receptor in immature rabbit epididymal cytosol. Endocrinology 105, 1128–1134 (1979)
Cornil, C.A., Ball, G.F., Balthazart, J.: Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 1126, 2–26 (2006)
Hess, R.A., Carnes, K.: The role of estrogen in testis and the male reproductive tract: a review and species comparison. Anim. Reprod. 1, 5–30 (2004)
Gruber, C.J., Tschugguel, W., Schneeberger, C., Huber, J.C.: Productions and actions of estrogens. N. Engl. J. Med. 346, 340–352 (2002)
Rupprecht, R., Holsboer, F.: Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 22, 410–416 (1999)
Falkenstein, E., Tillmann, H.-C., Christ, M., Feuring, M., Wehling, M.: Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol. Rev. 52, 513–555 (2000)
O'Boyle, N.M., Banck, M., James, C.A., Morley, C., Vandermeersch, T., Hutchison, G.R.: Open Babel: an open chemical toolbox. J. Cheminform. 3, 1–14 (2011)
Alizadeh-Rahrovi, J., Shayesteh, A., Ebrahim-Habibi, A.: Structural stability of myoglobin and glycomyoglobin: a comparative molecular dynamics simulation study. J. Biol. Phys. 41, 349–366 (2015)
Tautermann, C.S., Seeliger, D., Kriegl, J.M.: What can we learn from molecular dynamics simulations for GPCR drug design? Comput. Struct. Biotechnol. J. 13, 111–121 (2015)
Gajula, M.P., Kumar, A., Ijaq, J.: Protocol for molecular dynamics simulations of proteins. Bio-protocol. 6, e205 (2016)
Halgren, T.A.: MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 20, 720–729 (1999)
DeLano, W.L.: The PyMOL Molecular Graphics System (Version 1.8). DeLano Scientific LLC. Accessed in, San Carlos (2016)
Trott, O., Olson, O.J.: AutoDock Vina: improving speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461 (2010)
Abraham, M.J., van der Spoel, D., Lindahl, E., Hess, B.: GROMACS development team, GROMACS. www.gromacs.org, User manual version 5.0.2 (2014)
Zoete, V., Cuendet, M.A., Grosdidier, A., Michielin, O.: SwissParam, a fast force field generation tool for small organic molecules. J. Comput. Chem. 32, 2359–2368 (2011)
Ryckaert, J.P., Ciccotti, G., Berendsen, H.J.C.: Numeral integration of the Cartesian equation of motion of a system with constrains; molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977)
Miyamoto, S., Kollman, P.A.: SETTLE: an analytical version of the shake an RATTLE algorithms for molecular simulation. J. Comput. Chem. 13, 952–962 (1992)
Darden, T., York, D., Pedersen, L.J.: Particle mesh Ewald: an N·log (N) method for Ewald sums in large systems. J. Chem. Phys. 103, 8577–8593 (1993)
Salvalaglio, M., Perego, C., Giberti, F., Mazzotti, M., Parrinello, M.: Molecular-dynamics simulations of urea nucleation from aqueous solution. Proc. Natl. Acad. Sci. U.S.A. 112, E6–E14 (2015)
Anwar, M.A., Choi, S.: Structure-activity relationship in TLR4 mutations: atomistic molecular dynamics simulations and residue interaction network analysis. Sci. Rep. 7, 43807 (2017)
Shanbhag, V.P., Södergård, R.: The temperature dependence of the binding of 5α- dihydrotestosterone, testosterone and estradiol to the sex hormone globulin (SHBG) of human plasma. J. Steroid Biochem. 24, 549–555 (1986)
Hansson, T., Marelius, J., Åqvist, J.: Ligand binding affinity prediction by linear interaction energy methods. J. Comput. Aided Mol. Des. 12, 27–35 (1998)
Zwanzig, R.W.: High-temperature equation of state by a perturbation method. I. Nonpolar gases. J. Chem. Phys. 22, l1420–l1426 (1954)
Åqvist, J., Marelius, J.: The linear interaction energy method for predicting ligand binding free energies. Comb. Chem. High Throughput Screen. 4, 613–626 (2001)
von Hippel, P.H., Schleich, T.: In: Timasheff, S.N., Fasman, G.D. (eds.) Structure and Stability of Biological Macromolecules, pp. 416–574. Dekker, New York (1969)
Carter, J.E.A., Sluss, P.M.: Estradiol solubility in aqueous systems: effect of ionic concentrations, pH, and organic solvents. Journal of Hormones 2013, 1–4 (2013)
Ben-Naim, A.: The rise and fall of the hydrophobic effect in protein folding and protein-protein association and molecular recognition. Open J. Biophys. 1, 1–7 (2011)
Kim, J.S., Wu, Z., Morrow, A.R., Yethiraj, A., Yethiraj, A.: Self-diffusion and viscosity in electrolyte solutions. J. Phys. Chem. B 116, 12007–12013 (2012)
Ma, L., Cui, Q.: Temperature dependence of salt-protein association is sequence specific. Biochemistry 45, 14466–14472 (2006)
Ding, Y., Hassanali, A.A., Parrinello, M.: Anomalous water diffusion in salt solutions. Proc. Natl. Acad. Sci. U.S.A. 111, 3310–3315 (2014)
Rajamani, S., Ghosh, T., Garde, S.: Size dependent ion hydration, its asymmetry, and convergence to macroscopic behaviour. J. Chem. Phys. 120, 4457–4466 (2004)
Katzenellenbogen, J.A., Heiman, D.F., Carlson, K.E., Lloyd, J.E.: In vivo and in vitro steroid receptor assays in the design of estrogen pharmaceuticals. In: Eckelman, W.C. (ed.) Receptor-Binding Radiotracers, Vol. I, pp. 93–126. CRC, Florida (1982)
Chandra, A.: Static dielectric constant of aqueous electrolyte solutions: is there any dynamic contribution? J. Chem. Phys. 113, 903 (2000)
Li, Y., Girard, M., Shen, M., Millan, J.A., Cruz, M.O.: Strong attractions and repulsions mediated by monovalent salts. Proc. Natl. Acad. Sci. 114, 11838–11843 (2017)
Zasetsky, A.Y., Svishchev, I.M.: Dielectric response of concentrated NaCl aqueous solutions: molecular dynamics simulations. J. Chem. Phys. 115, 1448 (2001)
Anderson, J., Ullo, J., Yip, S.: Molecular dynamics simulation of the concentration-dependent dielectric constants of aqueous NaCl solutions. Chem. Phys. Lett. 152, 447–452 (1988)
Payne, V.A., Xu, J.-H., Forsyth, M., Ratner, M.A., Duward, F.S., Leeuw, S.W.: Clustering in molecular dynamics simulations of sodium iodide solutions. Electrochim. Acta 40, 2087–2091 (1995)
Payne, V.A., Xu, J.-H., Forsyth, M., Ratner, M.A., Duward, F.S., Leeuw, S.W.: Molecular dynamics simulations of ion clustering and conductivity in NaI/ether solutions. I. Effect of ion charge. J. Chem. Phys. 103, 8734–8745 (1995)
Payne, V.A., Xu, J.-H., Forsyth, M., Ratner, M.A., Duward, F.S., Leeuw, S.W.: Molecular dynamics simulations of ion clustering and conductivity in NaI/ether solutions. II. Effect of ion concentration. J. Chem. Phys. 103, 8746–8755 (1995)
Brodholt, J.P.: Molecular dynamics simulations of aqueous NaCl at high pressures and temperatures. Chem. Geol. 151, 11–19 (1998)
Friedman, R., Nachliel, E., Gutman, M.: Protein surface dynamics: interaction with water and small solutes. J. Biol. Phys. 31, 433–452 (2005)
Pfeiffer, S., Fushman, D., Cowburn, D.: Impact of Cl− and Na+ ions on simulated structure and dynamics of βARK1 PH domain. Proteins 35, 206–217 (1999)
Mokhdomi, T.A., Bukhari, S., Naveed, A.C., Amin, A., Wafai, A.H., Wani, S.H., Chowdri, N.A., Qadri, R.A.: A novel kinase mutation in VEGFR-1 predisposes its αC-helix/activation loop towards allosteric activation: atomic insights from protein simulation. Eur. J. Hum. Genet. 24, 1287–1293 (2016)
Ul-Haq, Z., Usmani, S., Iqbal, S., Zia, S.R.: In silico based investigation of dynamic variations in neprilysin (NEP and NEP2) proteins for extracting the point of specificity. Mol. BioSyst. 12, 1024–1036 (2016)
Ramharack, P., Oguntade, S., Soliman, M.E.S.: Delving into Zika virus structural dynamics—a closer look at NS3 helicase loop flexibility and its role in drug discovery. RSC Adv. 7, 22133–22144 (2017)
Aykut, A.O., Atilgan, A.R., Atilgan, C.: Designing molecular dynamics simulations to shift populations of the conformational states of calmodulin. PLoS Comput. Biol. 12, e1003366 (2013)
Gao, N., Liang, T., Yuan, Y., Xiao, X., Zhao, Y., Guo, Y., Li, M., Pu, X.: Exploring the mechanism of F282L mutation-caused constitutive activity of GPCR by a computational study. Phys. Chem. Chem. Phys. 18, 29412–29422 (2016)
Bye, J.W., Falconer, R.J.: Thermal stability of lysozyme as a function of ion concentration: a reappraisal of the relationship between the Hofmeister series and protein stability. Protein Sci. 22, 1563–1570 (2013)
Chen, C., Li, W.Z., Song, Y.C., Weng, L.D., Zhang, N.: Formation of water and glucose clusters by hydrogen bonds in glucose aqueous solutions. Comput. Theor. Chem. 984, 85–92 (2012)
Bulavin, L.A., Vergun, L.Y., Zabashta, Y.F., Teliman, E.O.: Large scaled clusters in aqueous glucose solutions. Colloid J. 77, 261–266 (2015)
Perry, J.R., Weedon, M.N., Langenberg, C., et al.: Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum. Mol. Genet. 19, 535–544 (2010)
Imamura, K., Ogawa, T., Sakiyama, T., Nakanishi, K.: Effects of types of sugar on the stabilization of protein in the dried state. J. Pharm. Sci. 92, 266–274 (2003)
Arakawa, T., Timasheff, S.N.: Stabilization of protein structure by sugars. Biochemistry 21, 6536–6544 (1982)
Wong, Y.-H., Tayyab, S.: Protein stabilizing potential of simulated honey sugar cocktail under various denaturation conditions. Process Biochem. 47, 1933–1943 (2012)
Ohan, M.P., Dunn, M.G.: Glucose stabilizes collagen sterilized with gamma irradiation. J. Biomed. Mater. Res. A 67, 1188–1195 (2003)
Lins, R.D., Pereira, C.S., Hünenberger, P.H.: Trehalose–protein interaction in aqueous solution. Proteins 55, 177–186 (2004)
Mittal, S., Chowhan, R.K., Rajendrakumar, L.: Macromolecular crowding: macromolecules friend or foe. Biochim. Biophys. Acta 1850, 1822–1831 (2015)
Waseda, Y., Ohtani, M.: Structure and effective interionic potential of liquid palladium, platinum and zirconium. Z. Physik B 21, 229–234 (1975)
Lyubartsev, A.P., Laaksonen, A.: Concentration effects in aqueous NaCl solutions. A molecular dynamics simulation. J. Phys. Chem. 100, 16410–16418 (1996)
Filipponi, A., DiCicco, A., Aquilanti, G., Minicucci, M., De Panfilis, S., Rybicki, J.: Short-range structure of liquid palladium and rhodium at very high temperatures. J. Non-Cryst. Solids 250-252, 172–176 (1999)
Balbuena, P.B., Johnston, K.P., Rossky, P.J.: Molecular dynamics simulation of electrolyte solutions in ambient and supercritical water. 1. Ion solvation. J. Phys. Chem. 100, 2706–2715 (1996)
Bocchinfuso, W.P., Ma, K.L., Lee, W.M., Warmels-Rodenhiser, S., Hammond, G.L.: Selective removal of glycosylation sites from sex hormone-binding globulin by site-directed mutagenesis. Endocrinology 131, 2331–2336 (1992)
Acknowledgements
We thank Prof. Márcio R. Maia and Cintia Garcia for reading the manuscript. We are also in debt to Prof. D.O.C. Santos for her valuable help during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 241 kb)
Rights and permissions
About this article
Cite this article
da Silva, A.J., dos Santos, E.S. Aqueous solution interactions with sex hormone-binding globulin and estradiol: a theoretical investigation. J Biol Phys 44, 539–556 (2018). https://doi.org/10.1007/s10867-018-9505-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-018-9505-8