Abstract
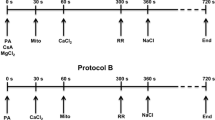
In cardiac mitochondria, matrix free Ca2+ ([Ca2+]m) is primarily regulated by Ca2+ uptake and release via the Ca2+ uniporter (CU) and Na+/Ca2+ exchanger (NCE) as well as by Ca2+ buffering. Although experimental and computational studies on the CU and NCE dynamics exist, it is not well understood how matrix Ca2+ buffering affects these dynamics under various Ca2+ uptake and release conditions, and whether this influences the stoichiometry of the NCE. To elucidate the role of matrix Ca2+ buffering on the uptake and release of Ca2+, we monitored Ca2+ dynamics in isolated mitochondria by measuring both the extra-matrix free [Ca2+] ([Ca2+]e) and [Ca2+]m. A detailed protocol was developed and freshly isolated mitochondria from guinea pig hearts were exposed to five different [CaCl2] followed by ruthenium red and six different [NaCl]. By using the fluorescent probe indo-1, [Ca2+]e and [Ca2+]m were spectrofluorometrically quantified, and the stoichiometry of the NCE was determined. In addition, we measured NADH, membrane potential, matrix volume and matrix pH to monitor Ca2+-induced changes in mitochondrial bioenergetics. Our [Ca2+]e and [Ca2+]m measurements demonstrate that Ca2+ uptake and release do not show reciprocal Ca2+ dynamics in the extra-matrix and matrix compartments. This salient finding is likely caused by a dynamic Ca2+ buffering system in the matrix compartment. The Na+- induced Ca2+ release demonstrates an electrogenic exchange via the NCE by excluding an electroneutral exchange. Mitochondrial bioenergetics were only transiently affected by Ca2+ uptake in the presence of large amounts of CaCl2, but not by Na+- induced Ca2+ release.
Similar content being viewed by others
References
Affolter H, Carafoli E (1980) The Ca2+-Na+ antiporter of heart mitochondria operates electroneutrally. Biochem Biophys Res Commun 95(1):193–196
Agarwal B, Camara AK, Stowe DF, Bosnjak ZJ, Dash RK (2012) Enhanced charge-independent mitochondrial free Ca2+ and attenuated ADP-induced NADH oxidation by isoflurane: Implications for cardioprotection. Biochim Biophys Acta 1817(3):453–465
Aldakkak M, Stowe DF, Cheng Q, Kwok WM, Camara AK (2010) Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening. Am J Physiol Cell Physiol 298(3):C530–C541
Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y et al (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476(7360):341–345
Baysal K, Jung DW, Gunter KK, Gunter TE, Brierley GP (1994) Na+-dependent Ca2+ efflux mechanism of heart mitochondria is not a passive Ca2+/2Na+ exchanger. Am J Physiol 266(3 Pt 1):C800–C808
Bazil JN, Blomeyer CA, Pradhan RK, Camara AK, & Dash RK (2012) Modeling the calcium sequestration system in isolated guinea pig cardiac mitochondria. J Bioenerg Biomembr, accepted for publication
Bernardi P, Rasola A (2007) Calcium and cell death: the mitochondrial connection. Subcell Biochem 45:481–506
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brand MD (1985) The stoichiometry of the exchange catalysed by the mitochondrial calcium/sodium antiporter. Biochem J 229(1):161–166
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287(4):C817–C833
Camara AK, Lesnefsky EJ, Stowe DF (2010) Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal 13(3):279–347
Camara AK, Bienengraeber M, Stowe DF (2011) Mitochondrial approaches to protect against cardiac ischemia and reperfusion injury. Front Physiol 2:13
Chalmers S, Nicholls DG (2003) The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem 278(21):19062–19070
Chinopoulos C, Adam-Vizi V (2010) Mitochondrial Ca2+ sequestration and precipitation revisited. FEBS J 277(18):3637–3651
Cox DA, Matlib MA (1993) A role for the mitochondrial Na+-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem 268(2):938–947
Crompton M, Heid I (1978) The cycling of calcium, sodium, and protons across the inner membrane of cardiac mitochondria. Eur J Biochem 91(2):599–608
Crompton M, Capano M, Carafoli E (1976) The sodium-induced efflux of calcium from heart mitochondria. Eur J Biochem 69(2):453–462
Dash RK, Beard DA (2008) Analysis of cardiac mitochondrial Na+-Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J Physiol 586(13):3267–3285
Dash RK, Qi F, Beard DA (2009) A biophysically based mathematical model for the kinetics of mitochondrial calcium uniporter. Biophys J 96(4):1318–1332
De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476(7360):336–340
Dedkova EN, Blatter LA (2008) Mitochondrial Ca2+ and the heart. Cell Calcium 44(1):77–91
Denton RM (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787(11):1309–1316
Genge BR, Wu LN, Wuthier RE (2007) In vitro modeling of matrix vesicle nucleation: synergistic stimulation of mineral formation by annexin A5 and phosphatidylserine. J Biol Chem 282(36):26035–26045
Graier WF, Frieden M, Malli R (2007) Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Arch 455(3):375–396
Griffiths EJ (2009) Mitochondrial calcium transport in the heart: physiological and pathological roles. J Mol Cell Cardiol 46(6):789–803
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6):3440–3450
Gunter TE, Pfeiffer DR (1990) Mechanisms by which mitochondria transport calcium. Am J Physiol 258(5 Pt 1):C755–C786
Gunter TE, Sheu SS (2009) Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. Biochim Biophys Acta 1787(11):1291–1308
Halestrap AP (2009) Mitochondria and reperfusion injury of the heart - a holey death but not beyond salvation. J Bioenerg Biomembr 41(2):113–121
Haumann J, Dash RK, Stowe DF, Boelens AD, Beard DA, Camara AK (2010) Mitochondrial free [Ca2+] increases during ATP/ADP antiport and ADP phosphorylation: exploration of mechanisms. Biophys J 99(4):997–1006
Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG et al (2007) Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol 293(3):H1400–H1407
Hoppe UC (2010) Mitochondrial calcium channels. FEBS Lett 584(10):1975–1981
Jung DW, Apel LM, Brierley GP (1992) Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. Am J Physiol 262(4 Pt 1):C1047–C1055
Jung DW, Baysal K, Brierley GP (1995) The sodium-calcium antiport of heart mitochondria is not electroneutral. J Biol Chem 270(2):672–678
Kaasik A, Safiulina D, Zharkovsky A, Veksler V (2007) Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol 292(1):C157–C163
Kim B, Matsuoka S (2008) Cytoplasmic Na+-dependent modulation of mitochondrial Ca2+ via electrogenic mitochondrial Na+-Ca2+ exchange. J Physiol 586(6):1683–1697
Li W, Shariat-Madar Z, Powers M, Sun X, Lane RD, Garlid KD (1992) Reconstitution, identification, purification, and immunological characterization of the 110-kDa Na+/Ca2+ antiporter from beef heart mitochondria. J Biol Chem 267(25):17983–17989
Nicholls DG, Chalmers S (2004) The integration of mitochondrial calcium transport and storage. J Bioenerg Biomembr 36(4):277–281
Olson ML, Chalmers S, McCarron JG (2012) Mitochondrial organization and Ca2+ uptake. Biochem Soc Trans 40(1):158–167
Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J et al (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 107(1):436–441
Paucek P, Jaburek M (2004) Kinetics and ion specificity of Na+/Ca2+ exchange mediated by the reconstituted beef heart mitochondrial Na+/Ca2+ antiporter. Biochim Biophys Acta 1659(1):83–91
Pradhan RK, Beard DA, Dash RK (2010a) A biophysically based mathematical model for the kinetics of mitochondrial Na+-Ca2+ antiporter. Biophys J 98(2):218–230
Pradhan RK, Qi F, Beard DA, Dash RK (2010b) Characterization of membrane potential dependency of mitochondrial Ca2+ uptake by an improved biophysical model of mitochondrial Ca2+ uniporter. PLoS One 5(10):e13278
Santo-Domingo J, Demaurex N (2010) Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 1797(6–7):907–912
Saotome M, Katoh H, Satoh H, Nagasaka S, Yoshihara S, Terada H et al (2005) Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 288(4):H1820–H1828
Scaduto RC Jr, Grotyohann LW (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J 76(1 Pt 1):469–477
Starkov AA (2010) The molecular identity of the mitochondrial Ca2+ sequestration system. FEBS J 277(18):3652–3663
Wei AC, Liu T, Cortassa S, Winslow RL, O’Rourke B (2011) Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A. Biochim Biophys Acta 1813(7):1373–1381
Wei AC, Liu T, Winslow RL, O’Rourke B (2012) Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol 139(6):465–478
Zoccarato F, Nicholls D (1982) The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria. Eur J Biochem 127(2):333–338
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1525 kb)
Rights and permissions
About this article
Cite this article
Blomeyer, C.A., Bazil, J.N., Stowe, D.F. et al. Dynamic buffering of mitochondrial Ca2+ during Ca2+ uptake and Na+-induced Ca2+ release. J Bioenerg Biomembr 45, 189–202 (2013). https://doi.org/10.1007/s10863-012-9483-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9483-7