Abstract
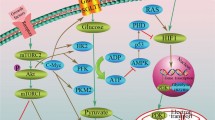
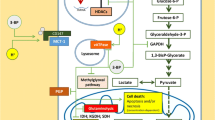
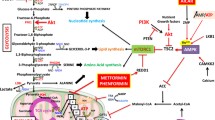
The Warburg effect refers to the phenomenon whereby cancer cells avidly take up glucose and produce lactic acid under aerobic conditions. Although the molecular mechanisms underlying tumor reliance on glycolysis remains not completely clear, its inhibition opens feasible therapeutic windows for cancer treatment. Indeed, several small molecules have emerged by combinatorial studies exhibiting promising anticancer activity both in vitro and in vivo, as a single agent or in combination with other therapeutic modalities. Therefore, besides reviewing the alterations of glycolysis that occur with malignant transformation, this manuscript aims at recapitulating the most effective pharmacological therapeutics of its targeting. In particular, we describe the principal mechanisms of action and the main targets of 3-bromopyruvate, an alkylating agent with impressive antitumor effects in several models of animal tumors. Moreover, we discuss the chemo-potentiating strategies that would make unparalleled the putative therapeutic efficacy of its use in clinical settings.
Similar content being viewed by others
References
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM (2003) P-glycoprotein: from genomics to mechanism. Oncogene 22(47):7468–7485. doi:10.1038/sj.onc.1206948
Apfel MA, Ikeda BH, Speckhard DC, Frey PA (1984) Escherichia coli pyruvate dehydrogenase complex. Thiamin pyrophosphate-dependent inactivation by 3-bromopyruvate. J Biol Chem 259(5):2905–2909
Arora KK, Pedersen PL (1988) Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem 263(33):17422–17428
Arzoine L, Zilberberg N, Ben-Romano R, Shoshan-Barmatz V (2009) Voltage-dependent anion channel 1-based peptides interact with hexokinase to prevent its anti-apoptotic activity. J Biol Chem 284(6):3946–3955. doi:10.1074/jbc.M803614200
Baker JP, Rabin BR (1969) Effects of bromopyruvate on the control and catalytic properties of glutamate dehydrogenase. Eur J Biochem 11(1):154–159
Barnard JP, Reynafarje B, Pedersen PL (1993) Glucose catabolism in African trypanosomes. Evidence that the terminal step is catalyzed by a pyruvate transporter capable of facilitating uptake of toxic analogs. J Biol Chem 268(5):3654–3661
Barnes DJ, Palaiologou D, Panousopoulou E, Schultheis B, Yong AS, Wong A et al (2005) Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res 65(19):8912–8919. doi:10.1158/0008-5472.CAN-05-0076
Bolanos JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35(3):145–149. doi:10.1016/j.tibs.2009.10.006
Boros LG, Puigjaner J, Cascante M, Lee WN, Brandes JL, Bassilian S et al (1997) Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res 57(19):4242–4248
Cambier N, Chopra R, Strasser A, Metcalf D, Elefanty AG (1998) BCR-ABL activates pathways mediating cytokine independence and protection against apoptosis in murine hematopoietic cells in a dose-dependent manner. Oncogene 16(3):335–348. doi:10.1038/sj.onc.1201490
Cao X, Bloomston M, Zhang T, Frankel WL, Jia G, Wang B et al (2008) Synergistic antipancreatic tumor effect by simultaneously targeting hypoxic cancer cells with HSP90 inhibitor and glycolysis inhibitor. Clin Cancer Res 14(6):1831–1839. doi:10.1158/1078-0432.CCR-07-1607
Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A et al (2011) Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med 3(94):94ra70. doi:10.1126/scitranslmed.3002394
Chang GG, Hsu RY (1973) The substrate analog bromopyruvate as a substrate, an inhibitor and an alkylating agent of malic enzyme of pigeon liver. Biochem Biophys Res Commun 55(3):580–587
Chen Z, Zhang H, Lu W, Huang P (2009) Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta 1787(5):553–560. doi:10.1016/j.bbabio.2009.03.003
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R et al (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452(7184):230–233. doi:10.1038/nature06734
Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L et al (2007) GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129(5):983–997. doi:10.1016/j.cell.2007.03.045
Colell A, Green DR, Ricci JE (2009) Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ 16(12):1573–1581. doi:10.1038/cdd.2009.137
Conroy CW, Maren TH (1985) The determination of osteopetrotic phenotypes by selective inactivation of red cell carbonic anhydrase isoenzymes. Clin Chim Acta 152(3):347–354
Culler MD, Oberg K, Arnold R, Krenning EP, Sevilla I, Diaz JA (2011) Somatostatin analogs for the treatment of neuroendocrine tumors. Cancer Metastasis Rev 30(Suppl 1):9–17. doi:10.1007/s10555-011-9293-0
da Pereira Silva AP, El-Bacha T, Kyaw N, dos Santos RS, da-Silva WS, Almeida FC et al (2009) Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem J 417(3):717–726. doi:10.1042/BJ20080805
De Lena M, Lorusso V, Latorre A, Fanizza G, Gargano G, Caporusso L et al (2001) Paclitaxel, cisplatin and lonidamine in advanced ovarian cancer. A phase II study Eur J Cancer 37(3):364–368
Dell’Antone P (2009) Targets of 3-bromopyruvate, a new, energy depleting, anticancer agent. Med Chem 5(6):491–496
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347(7):472–480. doi:10.1056/NEJMoa020461
Ditonno P, Battaglia M, Selvaggio O, Garofalo L, Lorusso V, Selvaggi FP (2005) Clinical Evidence Supporting the Role of Lonidamine for the Treatment of BPH. Rev Urol 7(Suppl 7):S27–33
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S et al (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 2(5):561–566
Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM et al (2001) Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 344(14):1038–1042. doi:10.1056/NEJM200104053441402
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N et al (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355(23):2408–2417. doi:10.1056/NEJMoa062867
Eigenbrodt E, Glossmann H (1980) Glycolysis-one of the keys to cancer? Pharmacol Sci 1(2):240–245. doi:doi:10.1016/0165-6147(80)90009-7
El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S, Munoz-Pinedo C (2011) Sugar-free approaches to cancer cell killing. Oncogene 30(3):253–264. doi:10.1038/onc.2010.466
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR et al (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64(11):3892–3899. doi:10.1158/0008-5472.CAN-03-2904
Fang J, Quinones QJ, Holman TL, Morowitz MJ, Wang Q, Zhao H et al (2006) The H + -linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol 70(6):2108–2115. doi:10.1124/mol.106.026245
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9(6):425–434. doi:10.1016/j.ccr.2006.04.023
Filomeni G, Rotilio G, Ciriolo MR (2005) Disulfide relays and phosphorylative cascades: partners in redox-mediated signaling pathways. Cell Death Differ 12(12):1555–1563. doi:10.1038/sj.cdd.4401754
Filomeni G, Desideri E, Cardaci S, Graziani I, Piccirillo S, Rotilio G et al (2010) Carcinoma cells activate AMP-activated protein kinase-dependent autophagy as survival response to kaempferol-mediated energetic impairment. Autophagy 6(2):202–216
Filomeni G, Cardaci S, Da Costa Ferreira AM, Rotilio G, Ciriolo MR (2011) Metabolic oxidative stress elicited by the copper(II) complex [Cu(isaepy)2] triggers apoptosis in SH-SY5Y cells through the induction of the AMP-activated protein kinase/p38MAPK/p53 signalling axis: evidence for a combined use with 3-bromopyruvate in neuroblastoma treatment. Biochem J 437(3):443–453. doi:10.1042/BJ20110510
Fonda ML (1976) Bromopyruvate inactivation of glutamate apodecarboxylase. Kinetics and specificity. J Biol Chem 251(1):229–235
Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S et al (2008) Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 10(1):193–199. doi:10.1208/s12248-008-9022-y
Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I et al (2009) Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res 29(12):4909–4918
Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Syed LH, Rao PP et al (2010) 3-Bromopyruvate induces endoplasmic reticulum stress, overcomes autophagy and causes apoptosis in human HCC cell lines. Anticancer Res 30(3):923–935
Gatzemeier U, Cavalli F, Haussinger K, Kaukel E, Koschel G, Martinelli G et al (1991) Phase III trial with and without lonidamine in non-small cell lung cancer. Semin Oncol 18(2 Suppl 4):42–48
Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL (2002) Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res 62(14):3909–3913
Goldman RD, Kaplan NO, Hall TC (1964) Lactic dehydrogenase in human neoplastic tissues. Cancer Res 24:389–399
Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C et al (1998) GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer 83(1):34–40
Halestrap AP, Wilson MC (2011) The monocarboxylate transporter family-role and regulation. IUBMB Life. doi:10.1002/iub.572.
Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y et al (2005) S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7(7):665–674. doi:10.1038/ncb1268
Hong C, Maunakea A, Jun P, Bollen AW, Hodgson JG, Goldenberg DD et al (2005) Shared epigenetic mechanisms in human and mouse gliomas inactivate expression of the growth suppressor SLC5A8. Cancer Res 65(9):3617–3623. doi:10.1158/0008-5472.CAN-05-0048
Hulleman E, Kazemier KM, Holleman A, VanderWeele DJ, Rudin CM, Broekhuis MJ et al (2009) Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood 113(9):2014–2021. doi:10.1182/blood-2008-05-157842
Ihrlund LS, Hernlund E, Khan O, Shoshan MC (2008) 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol 2(1):94–101. doi:10.1016/j.molonc.2008.01.003
Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D et al (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344(14):1052–1056. doi:10.1056/NEJM200104053441404
Kantarjian HM, Baccarani M, Jabbour E, Saglio G, Cortes JE (2011) Second generation tyrosine kinase inhibitors: the future of frontline CML therapy. Clin Cancer Res 17(7):1674–1683
Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY et al (2005) Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res 11(8):2785–2808. doi:10.1158/1078-0432.CCR-04-2626
Kim W, Yoon JH, Jeong JM, Cheon GJ, Lee TS, Yang JI et al (2007) Apoptosis-inducing antitumor efficacy of hexokinase II inhibitor in hepatocellular carcinoma. Mol Cancer Ther 6(9):2554–2562. doi:10.1158/1535-7163.MCT-07-0115
Kim JS, Ahn KJ, Kim JA, Kim HM, Lee JD, Lee JM et al (2008) Role of reactive oxygen species-mediated mitochondrial dysregulation in 3-bromopyruvate induced cell death in hepatoma cells: ROS-mediated cell death by 3-BrPA. J Bioenerg Biomembr 40(6):607–618. doi:10.1007/s10863-008-9188-0
Ko YH, Smith BL, Wang Y, Pomper MG, Rini DA, Torbenson MS et al (2004) Advanced cancers: eradication in all cases using 3-bromopyruvate therapy to deplete ATP. Biochem Biophys Res Commun 324(1):269–275. doi:10.1016/j.bbrc.2004.09.047
Kornberg MD, Sen N, Hara MR, Juluri KR, Nguyen JV, Snowman AM et al (2010) GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol 12(11):1094–1100. doi:10.1038/ncb2114
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL (2005) Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 7(1):1–6
Kroemer G, Pouyssegur J (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13(6):472–482. doi:10.1016/j.ccr.2008.05.005
Langbein S, Zerilli M, Zur Hausen A, Staiger W, Rensch-Boschert K, Lukan N et al (2006) Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 94(4):578–585. doi:10.1038/sj.bjc.6602962
Latz D, Thonke A, Juling-Pohlit L, Pohlit W (1993) Tumor response to ionizing radiation and combined 2-deoxy-D-glucose application in EATC tumor bearing mice: monitoring of tumor size and microscopic observations. Strahlenther Onkol 169(7):405–411
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM et al (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107(5):2037–2042. doi:10.1073/pnas.0914433107
Leiprecht N, Munoz C, Alesutan I, Siraskar G, Sopjani M, Foller M et al (2011) Regulation of Na(+)-coupled glucose carrier SGLT1 by human papillomavirus 18 E6 protein. Biochem Biophys Res Commun 404(2):695–700. doi:10.1016/j.bbrc.2010.12.044
Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L et al (2003) SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A 100(14):8412–8417. doi:10.1073/pnas.1430846100
Macchioni L, Davidescu M, Sciaccaluga M, Marchetti C, Migliorati G, Coaccioli S et al (2011) Mitochondrial dysfunction and effect of antiglycolytic bromopyruvic acid in GL15 glioblastoma cells. J Bioenerg Biomembr 43(5):507–518. doi:10.1007/s10863-011-9375-2
Macheda ML, Rogers S, Best JD (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol 202(3):654–662. doi:10.1002/jcp.20166
Mahraoui L, Rodolosse A, Barbat A, Dussaulx E, Zweibaum A, Rousset M et al (1994) Presence and differential expression of SGLT1, GLUT1, GLUT2, GLUT3 and GLUT5 hexose-transporter mRNAs in Caco-2 cell clones in relation to cell growth and glucose consumption. Biochem J 298(Pt 3):629–633
Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF et al (2004) 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64(1):31–34
Mathupala SP, Ko YH, Pedersen PL (2009) Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol 19(1):17–24. doi:10.1016/j.semcancer.2008.11.006
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43(7):969–980. doi:10.1016/j.biocel.2010.02.005
Mazurek S, Boschek CB, Hugo F, Eigenbrodt E (2005) Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 15(4):300–308. doi:10.1016/j.semcancer.2005.04.009
McClelland GB, Brooks GA (2002) Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J Appl Physiol 92(4):1573–1584. doi:10.1152/japplphysiol.01069.2001
Meloche HP, Monti CT, Hogue-Angeletti RA (1978) Identification of the bromopyruvate-sensitive glutamate within the active site of 2-keto-3-deoxygluconate-6-P aldolase. Biochem Biophys Res Commun 84(3):589–594
Messner KR, Imlay JA (2002) Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J Biol Chem 277(45):42563–42571. doi:10.1074/jbc.M204958200
Nelson JA, Falk RE (1993) Phloridzin and phloretin inhibition of 2-deoxy-D-glucose uptake by tumor cells in vitro and in vivo. Anticancer Res 13(6A):2293–2299
Neri D, Supuran CT (2011) Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10(10):767–777. doi:10.1038/nrd3554
Oudard S, Carpentier A, Banu E, Fauchon F, Celerier D, Poupon MF et al (2003) Phase II study of lonidamine and diazepam in the treatment of recurrent glioblastoma multiforme. J Neurooncol 63(1):81–86
Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3(3):187–197. doi:10.1016/j.cmet.2006.01.012
Paradies G, Capuano F, Palombini G, Galeotti T, Papa S (1983) Transport of pyruvate in mitochondria from different tumor cells. Cancer Res 43(11):5068–5071
Park JB, Levine M (2000) Intracellular accumulation of ascorbic acid is inhibited by flavonoids via blocking of dehydroascorbic acid and ascorbic acid uptakes in HL-60, U937 and Jurkat cells. J Nutr 130(5):1297–1302
Park JY, Helm JF, Zheng W, Ly QP, Hodul PJ, Centeno BA et al (2008) Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas 36(4):e32–39. doi:10.1097/MPA.0b013e3181630ffe
Pastorino JG, Shulga N, Hoek JB (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277(9):7610–7618. doi:10.1074/jbc.M109950200
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updat 7:97–110
Qin JZ, Xin H, Nickoloff BJ (2010) 3-Bromopyruvate induces necrotic cell death in sensitive melanoma cell lines. Biochem Biophys Res Commun 396(2):495–500. doi:10.1016/j.bbrc.2010.04.126
Ramirez-Peinado S, Alcazar-Limones F, Lagares-Tena L, El Mjiyad N, Caro-Maldonado A, Tirado OM et al (2011) 2-deoxyglucose induces noxa-dependent apoptosis in alveolar rhabdomyosarcoma. Cancer Res 71(21):6796–6806. doi:10.1158/0008-5472.CAN-11-0759
Rodriguez-Enriquez S, Marin-Hernandez A, Gallardo-Perez JC, Moreno-Sanchez R (2009) Kinetics of transport and phosphorylation of glucose in cancer cells. J Cell Physiol 221(3):552–559. doi:10.1002/jcp.21885
Rudlowski C, Becker AJ, Schroder W, Rath W, Buttner R, Moser M (2003) GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am J Clin Pathol 120(5):691–698. doi:10.1309/4KYN-QM58-62JW-2GD7
Saksena S, Theegala S, Bansal N, Gill RK, Tyagi S, Alrefai WA et al (2009) Mechanisms underlying modulation of monocarboxylate transporter 1 (MCT1) by somatostatin in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 297(5):G878–885
Sanborn BM, Felberg NT, Hollocher TC (1971) The inactivation of succinate dehydrogenase by bromopyruvate. Biochim Biophys Acta 227(2):219–231
Schaefer NG, Geschwind JF, Engles J, Buchanan JW, Wahl RL (2012) Systemic administration of 3-bromopyruvate in treating disseminated aggressive lymphoma. Transl Res 159(1):51–57. doi:10.1016/j.trsl.2011.08.008
Schek N, Hall BL, Finn OJ (1988) Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res 48(22):6354–6359
Schwaab J, Horisberger K, Strobel P, Bohn B, Gencer D, Kahler G et al (2011) Expression of Transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer 11:363. doi:10.1186/1471-2407-11-363
Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA et al (1997) c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A 94(13):6658–6663
Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR (2007) 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 67(7):3364–3370. doi:10.1158/0008-5472.CAN-06-3717
Singh D, Banerji AK, Dwarakanath BS, Tripathi RP, Gupta JP, Mathew TL et al (2005) Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol 181(8):507–514. doi:10.1007/s00066-005-1320-z
Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN et al (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118(12):3930–3942. doi:10.1172/JCI36843
Strobel P, Allard C, Perez-Acle T, Calderon R, Aldunate R, Leighton F (2005) Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem J 386(Pt 3):471–478. doi:10.1042/BJ20040703
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5(3):219–234. doi:10.1038/nrd1984
Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N et al (2007) GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene 26(18):2606–2620. doi:10.1038/sj.onc.1210074
Tennant DA, Duran RV, Gottlieb E (2010) Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10(4):267–277. doi:10.1038/nrc2817
Thangaraju M, Gopal E, Martin PM, Ananth S, Smith SB, Prasad PD et al (2006) SLC5A8 triggers tumor cell apoptosis through pyruvate-dependent inhibition of histone deacetylases. Cancer Res 66(24):11560–11564. doi:10.1158/0008-5472.CAN-06-1950
Thangaraju M, Karunakaran SK, Itagaki S, Gopal E, Elangovan S, Prasad PD et al (2009) Transport by SLC5A8 with subsequent inhibition of histone deacetylase 1 (HDAC1) and HDAC3 underlies the antitumor activity of 3-bromopyruvate. Cancer 115(20):4655–4666. doi:10.1002/cncr.24532
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–91
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281(14):9030–9037. doi:10.1074/jbc.M511397200
Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10(9):671–684. doi:10.1038/nrd3504
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Whitman SP, Hackanson B, Liyanarachchi S, Liu S, Rush LJ, Maharry K et al (2008) DNA hypermethylation and epigenetic silencing of the tumor suppressor gene, SLC5A8, in acute myeloid leukemia with the MLL partial tandem duplication. Blood 112(5):2013–2016. doi:10.1182/blood-2008-01-128595
Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R et al (2011) Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med 208(2):313–326. doi:10.1084/jem.20101470
Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS et al (2008) A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther 7(11):3546–3555. doi:10.1158/1535-7163.MCT-08-0569
Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P (2005a) Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia 19(12):2153–2158. doi:10.1038/sj.leu.2403968
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN et al (2005b) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65(2):613–621
Yang CM, Liu YZ, Liao JW, Hu ML (2010) The in vitro and in vivo anti-metastatic efficacy of oxythiamine and the possible mechanisms of action. Clin Exp Metastasis 27(5):341–349. doi:10.1007/s10585-010-9331-2
Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J (1996a) Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res 56(5):1164–1167
Younes M, Lechago LV, Lechago J (1996b) Overexpression of the human erythrocyte glucose transporter occurs as a late event in human colorectal carcinogenesis and is associated with an increased incidence of lymph node metastases. Clin Cancer Res 2(7):1151–1154
Yu SJ, Yoon JH, Lee JH, Myung SJ, Jang ES, Kwak MS et al (2011) Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol Sin 32(7):912–920. doi:10.1038/aps.2011.24
Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H et al (2009) Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325(5947):1555–1559. doi:10.1126/science.1174229
Zhang XD, Deslandes E, Villedieu M, Poulain L, Duval M, Gauduchon P et al (2006) Effect of 2-deoxy-D-glucose on various malignant cell lines in vitro. Anticancer Res 26(5A):3561–3566
Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. (2011) Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. doi:10.1158/0008-5472.CAN-11-1674.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cardaci, S., Desideri, E. & Ciriolo, M.R. Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J Bioenerg Biomembr 44, 17–29 (2012). https://doi.org/10.1007/s10863-012-9422-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9422-7