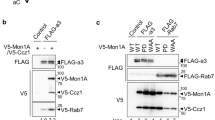
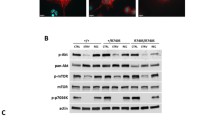
Vacuolar H+-ATPases (V-ATPases) are transported from cytosolic compartments to the ruffled plasma membrane of osteoclasts as they activate to resorb bone. Transport of V-ATPases is essential for bone resorption, and is associated with binding interactions between V-ATPases and microfilaments that are mediated by an actin-binding site in subunit B. This site is contained within 44 amino acids in the amino terminal domain, and requires a sequence motif that resembles an actin-binding motif found in mammalian profilin 1. Small alterations in the profilin-like sequence disrupt the actin-binding activity of subunit B. The interaction between V-ATPases and microfilaments in osteoclasts is regulated in response to changes in phosphatidylinositol-3 kinase activity. During internalization of V-ATPases from the plasma membrane of osteoclasts after a cycle of resorption, V-ATPases bind microfilaments that are in podosomes, dynamic actin-based structures, also present in metastatic cancer cells. Studies are ongoing to establish the physiological role of the microfilament-binding activity of subunit B in osteoclasts and in other cells.
Similar content being viewed by others
References
Biswas, R. S., Baker, D., Hruska, K. A., and Chellaiah, M. A. (2004). BMC Cell Biol. 5, 19.
Blair, H. C., Teitelbaum, S. L., Ghiselli, R., and Gluck, S. (1989). Science 245, 855–857.
Cao, H., Weller, S., Orth, J. D., Chen, J., Huang, B., Chen, J. L., Stamnes, M., and McNiven, M. A. (2005). Nat. Cell Biol. 7, 483–492.
Chellaiah, M. A., Biswas, R. S., Yuen, D., Alvarez, U. M., and Hruska, K. A. (2001). 276, 47434–47444.
Chen, S. H., Bubb, M. R., Yarmola, E. G., Zuo, J., Jiang, J., Lee, B. S., Lu, M., Gluck, S. L., Hurst, I. R., and Holliday, L. S. (2004). J. Biol. Chem. 279, 7988–7998.
DeMali, K. A., Wennerberg, K., and Burridge, K. (2003). Curr. Opin. Cell Biol. 15, 572–582.
Destaing, O., Saltel, F., Geminard, J. C., Jurdic, P., and Bard, F. (2003). Mol. Biol. Cell 14, 407–416.
Drory, O., Frolow, F., and Nelson, N. (2004). EMBO Rep. 5, 1148–1152.
Feng, X., Novack, D. V., Faccio, R., Ory, D. S., Aya, K., Boyer, M. I., McHugh, K. P., Ross, F. P., and Teitelbaum, S. L. (2001). J. Clin. Invest. 107, 1137–1144.
Gluck, S. L., Lee, B. S., Wang, S. P., Underhill, D., Nemoto, J., and Holliday, L. S. (1998). Acta Physiol. Scand. Suppl. 643, 203–212.
Gluck, S. L., Underhill, D. M., Iyori, M., Holliday, L. S., Kostrominova, T. Y., and Lee, B. S. (1996). Annu. Rev. Physiol. 58, 427–445.
Gundelfinger, E. D., Kessels, M. M., and Qualmann, B. (2003). Nat. Rev. Mol. Cell Biol. 4, 127–139.
Holliday, L. S., Lu, M., Lee, B. S., Nelson, R. D., Solivan, S., Zhang, L., and Gluck, S. L. (2000). J. Biol. Chem. 275, 32331–32337.
Holliday, L. S., Welgus, H. G., Fliszar, C. J., Veith, G. M., Jeffrey, J. J., and Gluck, S. L. (1997). J. Biol. Chem. 272, 22053–22058.
Holliday, L. S., Welgus, H. G., Hanna, J., Lee, B. S., Lu, M., Jeffrey, J. J., and Gluck, S. L. (2003). Calcif. Tissue Int. 72, 206–214.
Hurst, I. R., Zuo, J., Jiang, J., and Holliday, L. S. (2004). J. Bone Miner. Res. 19, 499–506.
King, G. J., and Holtrop, M. E. (1975). J. Cell Biol. 66, 445–451.
Lee, B. S., Gluck, S. L., and Holliday, L. S. (1999a). J. Biol. Chem. 274, 29164–29171.
Lee, B. S., Holliday, L. S., Krits, I., and Gluck, S. L. (1999b). J. Bone Miner. Res. 14, 2127–2136.
Linder, S., and Aepfelbacher, M. (2003). Trends Cell Biol. 13, 376–385.
Machesky, L. M., and Bornens, M. (2003). Curr. Opin. Cell Biol. 15, 2–5.
McHugh, K. P., Hodivala-Dilke, K., Zheng, M. H., Namba, N., Lam, J., Novack, D., Feng, X., Ross, F. P., Hynes, R. O., and Teitelbaum, S. L. (2000). J. Clin. Invest. 105, 433–440.
McNiven, M. A., Baldassarre, M., and Buccione, R. (2004). Front. Biosci. 9, 1944–1953.
Nakamura, I., Sasaki, T., Tanaka, S., Takahashi, N., Jimi, E., Kurokawa, T., Kita, Y., Ihara, S., Suda, T., and Fukui, Y. (1997). J. Cell Physiol. 172, 230–239.
Nishi, T., and Forgac, M. (2002). Nat. Rev. Mol. Cell Biol. 3, 94–103.
Ross, F. P., Chappel, J., Alvarez, J. I., Sander, D., Butler, W. T., Farach-Carson, M. C., Mintz, K. A., Robey, P. G., Teitelbaum, S. L., and Cheresh, D. A. (1993). J. Biol. Chem. 268, 9901–9907.
Saltel, F., Destaing, O., Bard, F., Eichert, D., and Jurdic, P. (2004). Mol. Biol. Cell 15, 5231–5241.
Schafer, D. A., Weed, S. A., Binns, D., Karginov, A. V., Parsons, J. T., and Cooper, J. A. (2002). Curr. Biol. 12, 1852–1857.
Schluter, K., Schleicher, M., and Jockusch, B. M. (1998). J. Cell Sci. 111(Pt 22), 3261–3273.
Smith, A. N., Borthwick, K. J., and Karet, F. E. (2002). Gene 297, 169–177.
Sun-Wada, G. H., Wada, Y., and Futai, M. (2004). Biochim. Biophys. Acta 1658, 106–114.
Teti, A., Barattolo, R., Grano, M., Colucci, S., Argentino, L., Teitelbaum, S. L., Hruska, K. A., Santacroce, G., and Zambonin, Z. A. (1989). Boll. Soc. Ital. Biol. Sper. 65, 1039–1043.
Toyomura, T., Oka, T., Yamaguchi, C., Wada, Y., and Futai, M. (2000). J. Biol. Chem. 275, 8760–8765.
Vaananen, H. K., Karhukorpi, E. K., Sundquist, K., Wallmark, B., Roininen, I., Hentunen, T., Tuukkanen, J., and Lakkakorpi, P. (1990). J. Cell Biol. 111, 1305–1311.
Vaananen, H. K., Zhao, H., Mulari, M., and Halleen, J. M. (2000). J. Cell Sci. 113(Pt 3), 377–381.
Vitavska, O., Merzendorfer, H., and Wieczorek, H. (2005). J. Biol. Chem. 280, 1070–1076.
Vitavska, O., Wieczorek, H., and Merzendorfer, H. (2003). J. Biol. Chem. 278, 18499–18505.
Wagner, C. A., Finberg, K. E., Breton, S., Marshansky, V., Brown, D., and Geibel, J. P. (2004). Physiol. Rev. 84, 1263–1314.
Weaver, A. M., Karginov, A. V., Kinley, A. W., Weed, S. A., Li, Y., Parsons, J. T., and Cooper, J. A. (2001). Curr. Biol. 11, 370–374.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holliday, L.S., Bubb, M.R., Jiang, J. et al. Interactions Between Vacuolar H+-ATPases and Microfilaments in Osteoclasts. J Bioenerg Biomembr 37, 419–423 (2005). https://doi.org/10.1007/s10863-005-9483-y
Issue Date:
DOI: https://doi.org/10.1007/s10863-005-9483-y