Abstract
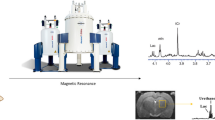
In vivo Nuclear Magnetic Resonance (NMR) spectroscopy has great potential to interpret the biochemical response of organisms to their environment, thus making it an essential tool in understanding toxic mechanisms. However, magnetic susceptibility distortions lead to 1D NMR spectra of living organisms with lines that are too broad to identify and quantify metabolites, necessitating the use of 2D 1H–13C Heteronuclear Single Quantum Coherence (HSQC) as a primary tool. While quantitative 2D HSQC is well established, to our knowledge it has yet to be applied in vivo. This study represents a simple pilot study that compares two of the most popular quantitative 2D HSQC approaches to determine if quantitative results can be directly obtained in vivo in isotopically enriched Daphnia magna (water flea). The results show the perfect-HSQC experiment performs very well in vivo, but the decoupling scheme used is critical for accurate quantitation. An improved decoupling approach derived using optimal control theory is presented here that improves the accuracy of metabolite concentrations that can be extracted in vivo down to micromolar concentrations. When combined with 2D Electronic Reference To access In vivo Concentrations (ERETIC) protocols, the protocol allows for the direct extraction of in vivo metabolite concentrations without the use of internal standards that can be detrimental to living organisms. Extracting absolute metabolic concentrations in vivo is an important first step and should, for example, be important for the parameterization as well as the validation of metabolic flux models in the future.




Similar content being viewed by others
References
Akhter M et al (2016) Identification of aquatically available carbon from algae through solution-state NMR of whole 13C-labelled cells. Anal Bioanal Chem 408:4357–4370
Akoka S, Barantin L, Trierweiler M (1999) Concentration measurement by proton NMR using the ERETIC method. Anal Chem 71:2554–2557
Anaraki MT, Simpson M, Simpson A (2018) Reducing impacts of organism variability in metabolomics via time trajectory in vivo NMR. Magn Reson Chem. https://doi.org/10.1002/mrc.4759
Aoto PC, Fenwick RB, Kroon GJA, Wright PE (2014) Accurate scoring of non-uniform sampling schemes for quantitative NMR. J Magn Reson 246:31–35
Bastawrous M, Jenne A, Tabatabaei Anaraki M, Simpson AJ (2018) In-vivo NMR spectroscopy: a powerful and complimentary tool for understanding environmental toxicity. Metabolites 8:1–24
Beauvoit BP et al (2017) Model-assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion model-assisted analysis of sugar metabolism througho tomato fruit development reveals enzyme and carrie. Plant Cell 2017:114
Bendall MR (1995) Broadband and narrowband spin decoupling using adiabatic spin flips. J Magn Reson 112:126–129
Bendall MR, Skinner TE (1998) Calibration of STUD + parameters to achieve optimally efficient broadband adiabatic decoupling in a single transient. J Magn Reson 134:331–349
Bingol K, Zhang F, Bruschweiler-Li L, Brüschweiler R (2013) Quantitative analysis of metabolic mixtures by two-dimensional 13C constant-time TOCSY NMR spectroscopy. Anal Chem 85:6414–6420
Bundy JG, Davey MP, Viant MR (2009) Environmental metabolomics: a critical review and future perspectives. Metabolomics 5:3–21
Burton IW, Quilliam MA, Walter JA (2005) Quantitative 1H NMR with external standards: use in preparation of calibration solutions for algal toxins and other natural products. Anal Chem 77:3123–3131
Castañar L, Parella T (2015) Recent advances in small molecule NMR: improved HSQC and HSQMBC experiments. Annu Rep NMR Spectrosc 84:163–232
Castañar L, Sistaré E, Virgili A, Williamson RT, Parella T (2015) Suppression of phase and amplitude J(HH) modulations in HSQC experiments. Magn Reson Chem 53:115–119
Colombié S et al (2015) Modelling central metabolic fluxes by constraint-based optimization reveals metabolic reprogramming of developing Solanum lycopersicum (tomato) fruit. Plant J 81:24–39
Di Gialleonardo V et al (2016) High-throughput indirect quantitation of 13C enriched metabolites using 1 H NMR. Anal Chem 88:11147–11153
Farjon J, Milande C, Martineau E, Akoka S, Giraudeau P (2018) The FAQUIRE approach: fast, quantitative, highly resolved and sensitivity enhanced 1H, 13C data. Anal Chem 90:1845–1851
Farooq H et al (2013) HR-MAS NMR spectroscopy: a practical guide for natural samples. Curr Org Chem 17:3013–3031
Fu R, Bodenhausen G (1995a) Ultra-broadband decoupling. J Magn Reson Ser A 117:324–325
Fu R, Bodenhausen G (1995b) Broadband decoupling in NMR with frequency-modulated ‘chirp’ pulses. Chem Phys Lett 245:415–420
Fu R, Bodenhausen G (1996) Evaluation of adiabatic frequency-modulated schemes for broadband decoupling in isotropic liquids. J Magn Reson Ser A 119:129–133
Fugariu I, Bermel W, Lane D, Soong R, Simpson AJ (2017) In-phase ultra high-resolution in vivo NMR. Angew Chem Int Ed 56:6324–6328
Giraudeau P, Akoka S (2013) Fast and ultrafast quantitative 2D NMR. Vital tools for efficient metabolomic approaches. In: Advances in botanical research, Elsevier, Amsterdam
Gronwald W et al (2008) Urinary metabolite quantification employing 2D NMR spectroscopy. Anal Chem 80:9288–9297
Heikkinen S, Toikka MM, Karhunen PT, Kilpeläinen IA (2003) Quantitative 2D HSQC (Q-HSQC) via suppression of J-dependence of polarization transfer in NMR spectroscopy: application to wood lignin. J Am Chem Soc 125:4362–4367
Hertkorn N et al (2007) High-precision frequency measurements: indispensable tools at the core of the molecular-level analysis of complex systems. Anal Bioanal Chem 389:1311–1327
Hu K, Westler WM, Markley JL (2011) Simultaneous quantification and identification of individual chemicals in metabolite mixtures by two-dimensional extrapolated time-zero 1H-13C HSQC (HSQC 0). J Am Chem Soc 133:1662–1665
Jézéquel T et al (2015) Absolute quantification of metabolites in tomato fruit extracts by fast 2D NMR. Metabolomics 11:1231–1242
Koskela H (2009) Chapter 1 quantitative 2D NMR studies. In: Annual reports on NMR spectroscopy, Elsevier, Amsterdam
Koskela H, Kilpeläinen I, Heikkinen S (2005) Some aspects of quantitative 2D NMR. J Magn Reson 174:237–244
Koskela H, Heikkilä O, Kilpeläinen I, Heikkinen S (2010) Quantitative two-dimensional HSQC experiment for high magnetic field NMR spectrometers. J Magn Reson 202:24–33
Kupče Ē, Freeman R (1995) Adiabatic pulses for wideband inversion and broadband decoupling. J Magn Reson Ser A 115:273–276
Lam B, Simpson AJ (2008) Direct 1H NMR spectroscopy of dissolved organic matter in natural waters. Analyst 133:263–269
Lewis IA et al (2007) Method for determining molar concentrations of metabolites in method for determining molar concentrations of metabolites in complex solutions from two-dimensional 1H-13C NMR spectra. 79:9385–9390
Mahrous EA, Farag MA (2015) Two dimensional NMR spectroscopic approaches for exploring plant metabolome: a review. J Adv Res 6:3–15
Majumdar RD et al (2017) In vivo solution-state NMR-based environmental metabolomics. Magn Res 6:133–148
Marchand J, Martineau E, Guitton Y, Dervilly-Pinel G, Giraudeau P (2017) Multidimensional NMR approaches towards highly resolved, sensitive and high-throughput quantitative metabolomics. Curr Opin Biotechnol 43:49–55
Martineau E, Tea I, Akoka S, Giraudeau P (2012) Absolute quantification of metabolites in breast cancer cell extracts by quantitative 2D 1H inadequate NMR. NMR Biomed 25:985–992
Martineau E, Akoka S, Boisseau R, Delanoue B, Giraudeau P (2013) Fast quantitative 1H-13C two-dimensional NMR with very high precision. Anal Chem 85:4777–4783
Mauve C, Khlifi S, Gilard F, Mouille G, Farjon J (2016) Sensitive, highly resolved, and quantitative 1H-13 C NMR data in one go for tracking metabolites in vegetal extracts. Chem Commun 52:6142–6145
Michel N, Akoka S, Michel N, Akoka S (2004) The application of the ERETIC method to 2D- NMR. J Magn Reson. https://doi.org/10.1016/j.jmr.2004.02.006
Mobarhan YL et al (2016) Comprehensive multiphase NMR applied to a living organism. Chem Sci 7:4856–4866
Morris GA, Freeman R (1979) Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc 101:760–762
Nagato EG et al (2013) 1H NMR-based metabolomics investigation of daphnia magna responses to sub-lethal exposure to arsenic, copper and lithium. Chemosphere 93:331–337
Neves JL, Heitmann B, Khaneja N, Glaser SJ (2009) Heteronuclear decoupling by optimal tracking. J Magn Reson 201:7–17
Nielsen J, Oliver S (2005) The next wave in metabolome analysis. Trends Biotechnol 23:544–546
Pathan M, Akoka S, Tea I, Charrier B, Giraudeau P (2011) ‘Multi-scan single shot’ quantitative 2D NMR: a valuable alternative to fast conventional quantitative 2D NMR. Analyst 136:3157–3163
Pauli GF, Gödecke T, Jaki BU, Lankin DC (2012) Quantitative 1 H NMR. development and potential of an analytical method: an update. J Nat Prod 75:834–851
Peterson DJ, Loening NM (2007) QQ-HSQC: a quick, quantitative heteronuclear correlation experiment for NMR spectroscopy. Magn Reson Chem 45:937–941
Schilling F et al (2014) Next-generation heteronuclear decoupling for high-field biomolecular NMR spectroscopy. Angew Chem Int Ed 53:4475–4479
Shaka AJ, Barker PB, Freeman R (1985) Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson 64:547–552
Sharma R, Gogna N, Singh H, Dorai K (2017) Fast profiling of metabolite mixtures using chemometric analysis of a speeded-up 2D heteronuclear correlation NMR experiment. RSC Adv 7:29860–29870
Simpson AJ, Liaghati Y, Fortier-McGill B, Soong R, Akhter M (2015) Perspective: in vivo NMR—a potentially powerful tool for environmental research. Magn Reson Chem 53:686–690
Skinner TE, Reiss TO, Luy B, Khaneja N, Glaser SJ (2003) Application of optimal control theory to the design of broadband excitation pulses for high-resolution NMR. J Magn Reson 163:8–15
Skinner TE et al (2006) Optimal control design of constant amplitude phase-modulated pulses: application to calibration-free broadband excitation. J Magn Reson 179:241–249
Soong R et al (2015) In vivo NMR spectroscopy: toward real time monitoring of environmental stress. Magn Reson Chem 53:774–779
Starčuk Z, Bartušek K, Starčuk Z (1994) Heteronuclear broadband spin-flip decoupling with adiabatic pulses. J Magn Reson Ser A 107:24–31
Tabatabaei Anaraki M et al (2018) Development and application of a low-volume flow system for solution-state in vivo NMR. Anal Chem 90:7912–7921
Tredwell GD, Behrends V, Geier FM, Liebeke M, Bundy JG (2011) Between-person comparison of metabolite fitting for NMR-based quantitative metabolomics. Anal Chem 83:8683–8687
Vuister W, Bax AD (1991) Letters to the editor. Tempo 435:69
Watanabe R et al (2016) Quantitative nuclear magnetic resonance spectroscopy based on PULCON methodology: application to quantification of invaluable marine toxin, okadaic acid. Toxins 8:1–9
Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM (2006) Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem 78:4430–4442
Wheeler HL et al (2015) Comprehensive multiphase NMR: a promising technology to study plants in their native state. Magn Reson Chem 53:735–744
Wider G, Dreier L (2006) Measuring protein concentrations by NMR spectroscopy. J Am Chem Soc 128:2571–2576
Yuk J, McKelvie JR, Simpson MJ, Spraul M, Simpson AJ (2010) Comparison of 1-D and 2-D NMR techniques for screening earthworm responses to sub-lethal endosulfan exposure. Environ Chem 7:524–536
Yuk J, Simpson MJ, Simpson AJ (2013) 1-D and 2-D NMR-based metabolomics of earthworms exposed to endosulfan and endosulfan sulfate in soil. Environ Pollut 175:35–44
Acknowledgements
We would like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic (STPGP 494273-16) and Discovery Programs (RGPIN-2014-05423), the Canada Foundation for Innovation (CFI), the Ontario Ministry of Research and Innovation (MRI), and the Krembil Foundation for providing funding. A. S. would like to thank the Government of Ontario for an Early Researcher Award. TES gratefully acknowledges support from the National Science Foundation under Grant No. CHE-1214006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lane, D., Skinner, T.E., Gershenzon, N.I. et al. Assessing the potential of quantitative 2D HSQC NMR in 13C enriched living organisms. J Biomol NMR 73, 31–42 (2019). https://doi.org/10.1007/s10858-018-0221-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-018-0221-2