Abstract
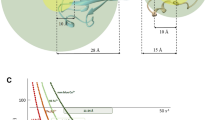
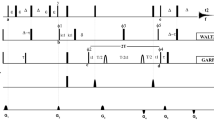
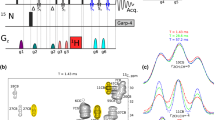
Paramagnetic relaxation enhancement (PRE) measurements constitute a powerful approach for detecting both permanent and transient protein–protein interactions. Typical PRE experiments require an intrinsic or engineered paramagnetic site on one of the two interacting partners; while a second, diamagnetic binding partner is labeled with stable isotopes (15N or 13C). Multiple paramagnetic labeled centers or reversed labeling schemes are often necessary to obtain sufficient distance restraints to model protein–protein complexes, making this approach time consuming and expensive. Here, we show a new strategy that combines a modified pulse sequence (1HN-Γ2-CCLS) with an asymmetric labeling scheme to enable the detection of both intra- and inter-molecular PREs simultaneously using only one sample preparation. We applied this strategy to the non-covalent dimer of ubiquitin. Our method confirmed the previously identified binding interface for the transient di-ubiquitin complex, and at the same time, unveiled the internal structural dynamics rearrangements of ubiquitin upon interaction. In addition to reducing the cost of sample preparation and speed up PRE measurements, by detecting the intra-molecular PRE this new strategy will make it possible to measure and calibrate inter-molecular distances more accurately for both symmetric and asymmetric protein–protein complexes.





Similar content being viewed by others
References
Banci L et al (2004) Paramagnetism-based restraints for Xplor-NIH. J Biomol NMR 28:249–261
Battiste JL, Wagner G (2000) Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry 39:5355–5365
Bernini A, Venditti V, Spiga O, Niccolai N (2009) Probing protein surface accessibility with solvent and paramagnetic molecules. Prog Nucl Magn Reson Spectrosc 54:278–289
Bertini I et al (2001a) Paramagnetism-based versus classical constraints: an analysis of the solution structure of Ca Ln calbindin D9k. J Biomol NMR 21:85–98
Bertini I, Luchinat C, Piccioli M (2001b) Paramagnetic probes in metalloproteins. Methods Enzymol 339:314–340
Bloembergen N, Morgan LO (1961) Proton relaxation times in paramagnetic solutions. Effects of electron spin relaxation. J Chem Phys 34:842–850
Brutscher B et al (2015) NMR methods for the study of instrinsically disordered proteins structure, dynamics, and interactions: general overview and practical guidelines. Adv Exp Med Biol 870:49–122
Chao FA, Shi L, Masterson LR, Veglia G (2012) FLAMEnGO: a fuzzy logic approach for methyl group assignment using NOESY and paramagnetic relaxation enhancement data. J Magn Reson 214:103–110
Clore GM (2008) Visualizing lowly-populated regions of the free energy landscape of macromolecular complexes by paramagnetic relaxation enhancement. Mol Biosyst 4:1058–1069
Clore GM, Iwahara J (2009) Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes. Chem Rev 109:4108–4139
Clore GM, Tang C, Iwahara J (2007) Elucidating transient macromolecular interactions using paramagnetic relaxation enhancement. Curr Opin Struct Biol 17:603–616
Dominguez C, Boelens R, Bonvin AM (2003) HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc 125:1731–1737
Emsley L, Bodenhausen G (1992) Optimization of shaped selective pulses for Nmr using a quaternion description of their overall propagators. J Magn Reson 97:135–148
Iwahara J, Clore GM (2010) Structure-independent analysis of the breadth of the positional distribution of disordered groups in macromolecules from order parameters for long, variable-length vectors using NMR paramagnetic relaxation enhancement. J Am Chem Soc 132:13346–13356
Iwahara J, Schwieters CD, Clore GM (2004) Ensemble approach for NMR structure refinement against (1)H paramagnetic relaxation enhancement data arising from a flexible paramagnetic group attached to a macromolecule. J Am Chem Soc 126:5879–5896
Iwahara J, Tang C, Clore GM (2007) Practical aspects of (1)H transverse paramagnetic relaxation enhancement measurements on macromolecules. J Magn Reson 184:185–195
Kosen PA (1989) Spin labeling of proteins. Methods Enzymol 177:86–121
Lange OF et al (2008) Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science 320:1471–1475
Liu Z et al (2012) Noncovalent dimerization of ubiquitin. Angew Chem Int Ed Engl 51:469–472
Liu Z et al. (2015) Lys63-linked ubiquitin chain adopts multiple conformational states for specific target recognition. Elife 4
Masterson LR, Tonelli M, Markley JL, Veglia G (2008) Simultaneous detection and deconvolution of congested NMR spectra containing three isotopically labeled species. J Am Chem Soc 130:7818–7819
Newby FN et al (2015) Structure-free validation of residual dipolar coupling and paramagnetic relaxation enhancement measurements of disordered proteins. Biochemistry 54:6876–6886
Nooren IM, Thornton JM (2003a) Diversity of protein–protein interactions. EMBO J 22:3486–3492
Nooren IM, Thornton JM (2003b) Structural characterisation and functional significance of transient protein–protein interactions. J Mol Biol 325:991–1018
Salmon L et al (2010) NMR characterization of long-range order in intrinsically disordered proteins. J Am Chem Soc 132:8407–8418
Salmon L, Bouvignies G, Markwick P, Blackledge M (2011) Nuclear magnetic resonance provides a quantitative description of protein conformational flexibility on physiologically important time scales. Biochemistry 50:2735–2747
Schmitz C, Bonvin AM (2011) Protein-protein HADDocking using exclusively pseudocontact shifts. J Biomol NMR 50:263–266
Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM (2003) The Xplor-NIH NMR molecular structure determination package. J Magn Reson 160:65–73
Solomon I (1955) Relaxation processes in a system of two spins. Phys Rev 99:559–565
Tonelli M, Masterson LR, Hallenga K, Veglia G, Markley JL (2007) Carbonyl carbon label selective (CCLS) 1H–15N HSQC experiment for improved detection of backbone 13C–15N cross peaks in larger proteins. J Biomol NMR 39:177–185
Tonelli M, Masterson LR, Cornilescu G, Markley JL, Veglia G (2009) One-sample approach to determine the relative orientations of proteins in ternary and binary complexes from residual dipolar coupling measurements. J Am Chem Soc 131:14138–14139
van Zundert GC, Melquiond AS, Bonvin AM (2015) Integrative modeling of biomolecular complexes: HADDOCKing with cryo-electron microscopy data. Structure 23:949–960
Venditti V, Fawzi NL (2018) Probing the atomic structure of transient protein contacts by paramagnetic relaxation enhancement solution NMR. In: Ghose R (ed) Protein NMR: methods and protocols. Springer, New York, pp 243–255
Walters KJ et al (2001) Characterizing protein-protein complexes and oligomers by nuclear magnetic resonance spectroscopy. Methods Enzymol 339:238–258
Zuiderweg ER (2002) Mapping protein-protein interactions in solution by NMR spectroscopy. Biochemistry 41:1–7
Acknowledgements
This research was supported by the National Institute of Health (GM 100310 and 1S10OD021536 to G.V.). We authors would like to thank Dr. G. Li for helping in the initial setting of the experiments. The experiments were carried out at the Minnesota NMR Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Olivieri, C., Subrahmanian, M.V., Xia, Y. et al. Simultaneous detection of intra- and inter-molecular paramagnetic relaxation enhancements in protein complexes. J Biomol NMR 70, 133–140 (2018). https://doi.org/10.1007/s10858-018-0165-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-018-0165-6