Abstract
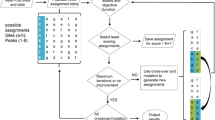
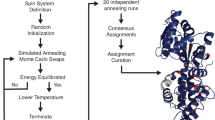
A multi-objective genetic algorithm is introduced to predict the assignment of protein solid-state NMR (SSNMR) spectra with partial resonance overlap and missing peaks due to broad linewidths, molecular motion, and low sensitivity. This non-dominated sorting genetic algorithm II (NSGA-II) aims to identify all possible assignments that are consistent with the spectra and to compare the relative merit of these assignments. Our approach is modeled after the recently introduced Monte-Carlo simulated-annealing (MC/SA) protocol, with the key difference that NSGA-II simultaneously optimizes multiple assignment objectives instead of searching for possible assignments based on a single composite score. The multiple objectives include maximizing the number of consistently assigned peaks between multiple spectra (“good connections”), maximizing the number of used peaks, minimizing the number of inconsistently assigned peaks between spectra (“bad connections”), and minimizing the number of assigned peaks that have no matching peaks in the other spectra (“edges”). Using six SSNMR protein chemical shift datasets with varying levels of imperfection that was introduced by peak deletion, random chemical shift changes, and manual peak picking of spectra with moderately broad linewidths, we show that the NSGA-II algorithm produces a large number of valid and good assignments rapidly. For high-quality chemical shift peak lists, NSGA-II and MC/SA perform similarly well. However, when the peak lists contain many missing peaks that are uncorrelated between different spectra and have chemical shift deviations between spectra, the modified NSGA-II produces a larger number of valid solutions than MC/SA, and is more effective at distinguishing good from mediocre assignments by avoiding the hazard of suboptimal weighting factors for the various objectives. These two advantages, namely diversity and better evaluation, lead to a higher probability of predicting the correct assignment for a larger number of residues. On the other hand, when there are multiple equally good assignments that are significantly different from each other, the modified NSGA-II is less efficient than MC/SA in finding all the solutions. This problem is solved by a combined NSGA-II/MC algorithm, which appears to have the advantages of both NSGA-II and MC/SA. This combination algorithm is robust for the three most difficult chemical shift datasets examined here and is expected to give the highest-quality de novo assignment of challenging protein NMR spectra.









Similar content being viewed by others
References
Baran MC, Huang YJ, Moseley HNB, Montelione GT (2004) Automated analysis of protein NMR assignments and structures. Chem Rev 104:3541–3555
Bartels C, Billeter M, Guntert P, Wuthrich K (1996) Automated sequence-specific NMR assignment of homologous proteins using the program GARANT. J Biomol NMR 7:207–213
Bertini I, Bhaumik A, De Paëpe G, Griffin RG, Lelli M, Lewandowski JR, Luchinat C (2010) High-resolution solid-state NMR structure of a 17.6 kDa protein. J Am Chem Soc 132:1032–1040
Böckmann A, Lange A, Galinier A, Luca S, Giraud N, Juy M, Heise H, Montserret R, Penin F, Baldus M (2003) Solid state NMR sequential resonance assignments and conformational analysis of the 2 × 10.4 kDa dimeric form of the Bacillus subtilis protein Crh. J Biomol NMR 27:323–339
Buchler NEG, Zuiderweg ERP, Wang H, Goldstein RA (1997) Protein heteronuclear NMR assignments using mean-field simulated annealing. J Magn Reson 125:34–42
Castellani F, vanRossum B, Diehl A, Schubert M, Rehbein K, Oschkinat H (2002) Structure of a protein determined by solid-state magic-angle spinning NMR spectroscopy. Nature 420:98–102
Coggins BE, Zhou P (2003) PACES: protein sequential assignment by computer-assisted exhaustive search. J Biomol NMR 26:93–111
Comellas G, Rienstra CM (2013) Protein structure determination by magic-angle spinning solid-state NMR, and insights into the formation, structure, and stability of amyloid fibrils. Annu Rev Biophys 42:515–536
Deb K, Pratap A, Agarwal S, Meyarivan T (2002) A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans Evol Comput 6:182–197
Franks WT, Zhou DH, Wylie BJ, Money BG, Graesser DT, Frericks HL, Sahota G, Rienstra CM (2005) Magic-angle spinning solid-state NMR spectroscopy of the beta1 immunoglobulin binding domain of protein G (GB1): 15N and 13C chemical shift assignments and conformational analysis. J Am Chem Soc 127:12291–12305
Franks WT, Wylie BJ, Schmidt HL, Nieuwkoop AJ, Mayrhofer RM, Shah GJ, Graesser DT, Rienstra CM (2008) Dipole tensor-based atomic-resolution structure determination of a nanocrystalline protein by solid-state NMR. Proc Natl Acad Sci USA 105:4621–4626
Fritzsching KJ, Yang Y, Schmidt-Rohr K, Hong M (2013) Practical use of chemical shift databases for protein solid-state NMR: 2D chemical shift maps and amino-acid assignment with secondary-structure information. J Biomol NMR 56:155–167
Hong M, Zhang Y, Hu F (2012) Membrane protein structure and dynamics from NMR spectroscopy. Annu Rev Phys Chem 63:1–24
Hu KN, McGlinchey RP, Wickner RB, Tycko R (2011a) Segmental polymorphism in a functional amyloid. Biophys J 101:2242–2250
Hu KN, Qiang W, Tycko R (2011b) A general Monte Carlo/simulated annealing algorithm for resonance assignment in NMR of uniformly labeled biopolymers. J Biomol NMR 50:267–276
Hyberts SG, Wagner G (2003) IBIS—a tool for automated sequential assignment of protein spectra from triple resonance experiments. J Biomol NMR 26:335–344
Igumenova TI, McDermott AE, Zilm KW, Martin RW, Paulson EK, Wand AJ (2004) Assignments of carbon NMR resonances for microcrystalline ubiquitin. J Am Chem Soc 126:6720–6727
Knowles JD, Corne DW (2000) Approximating the nondominated front using the Pareto archived evolution strategy. Evol Comput 8:149–172
Lee W, Yu W, Kim S, Chang I, Lee W, Markley JL (2012) PACSY, a relational database management system for protein structure and chemical shift analysis. J Biomol NMR 54:169–179
Leutner M, Gschwind RM, Liermann J, Schwarz C, Gemmecker G, Kessler H (1998) Automated backbone assignment of labeled proteins using the threshold accepting algorithm. J Biomol NMR 11:31–43
Li Y, Berthold DA, Gennis RB, Rienstra CM (2008) Chemical shift assignment of the transmembrane helices of DsbB, a 20-kDa integral membrane enzyme, by 3D magic-angle spinning NMR spectroscopy. Protein Sci 17:199–204
Li S, Zhang Y, Hong M (2010) 3D 13C–13C–13C correlation NMR for de novo distance determination of solid proteins and application to a human alpha defensin. J Magn Reson 202:203–210
Loquet A, Sgourakis NG, Gupta R, Giller K, Riedel D, Goosmann C, Griesinger C, Kolbe M, Baker D, Becker S, Lange A (2012) Atomic model of the type III secretion system needle. Nature 486:276–279
Luca S, Heise H, Baldus M (2003) High-resolution solid-state NMR applied to polypeptides and membrane proteins. Acc Chem Res 36:858–865
McDermott AE (2009) Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys 38:385–403
Moseley HNB, Monleon D, Montelione GT (2001) Automatic determination of protein backbone resonance assignments from triple resonance nuclear magnetic resonance data. Methods Enzymol 339:91–108
Olson JB, Markley JL (1994) Evaluation of an algorithm for the automated sequential assignment of protein backbone resonances: a demonstration of the connectivity tracing assignment tools (CONTRAST) software package. J Biomol NMR 4:385–410
Schmidt E, Guntert P (2012) A new algorithm for reliable and general NMR resonance assignment. J Am Chem Soc 134:12817–12829
Shi L, Ahmed MA, Zhang W, Whited G, Brown LS, Ladizhansky V (2009) Three-dimensional solid-state NMR study of a seven-helical integral membrane proton pump—structural insights. J Mol Biol 386:1078–1093
Shi L, Kawamura I, Jung KH, Brown LS, Ladizhansky V (2011) Conformation of a seven-helical transmembrane photosensor in the lipid environment. Angew Chem Int Ed Engl 50:1302–1305
Tycko R (2011) Solid-state NMR studies of amyloid fibril structure. Annu Rev Phys Chem 62:279–299
Tycko R, Hu KN (2010) A Monte Carlo/simulated annealing algorithm for sequential resonance assignment in solid state NMR of uniformly labeled proteins with magic-angle spinning. J Magn Reson 205:304–314
Wasmer C, Lange A, Van Melckebeke H, Siemer AB, Riek R, Meier BH (2008) Amyloid fibrils of the HET-s(218–289) prion form a beta solenoid with a triangular hydrophobic core. Science 319:1523–1526
Zhang Y, Doherty T, Li J, Lu W, Barinka C, Lubkowski J, Hong M (2010) Resonance assignment and three-dimensional structure determination of a human alpha-defensin, HNP-1, by solid-state NMR. J Mol Biol 397:408–422
Acknowledgments
This work is supported by NIH Grants GM088204 and GM066976. We thank Dr. Robert Tycko for helpful discussions and for making the MCASSIGN2 program available, and Myungwoon Lee for help in the initial phase of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Fritzsching, K.J. & Hong, M. Resonance assignment of the NMR spectra of disordered proteins using a multi-objective non-dominated sorting genetic algorithm. J Biomol NMR 57, 281–296 (2013). https://doi.org/10.1007/s10858-013-9788-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-013-9788-9