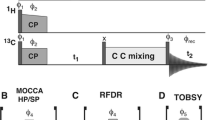
Abstract
Isotropic mixing sequences are one of the key methods to achieve efficient coherence transfer. Among them, the MOCCA-XY16, which keeps the magnetization longitudinal for a significant amount of time, is characterised by favourable relaxation properties. We show here that its adapted version is particularly suited for carbonyl–carbonyl correlations in 13C direct detection NMR experiments.






Similar content being viewed by others
References
Arnesano F, Banci L, Bertini I, Felli IC, Luchinat C, Thompsett AR (2003) A strategy for the NMR characterization of type II copper(II) proteins: the case of the copper trafficking protein CopC from Pseudomonas syringae. J Am Chem Soc 125:7200–7208
Babini E, Bertini I, Capozzi F, Felli IC, Lelli M, Luchinat C (2004) Direct carbon detection in paramagnetic metalloproteins to further exploit pseudocontact shift restraints. J Am Chem Soc 126:10496–10497
Balayssac S, Jiménez B, Piccioli M (2006) 13C Direct Detected COCO-TOCSY: a tool for sequence specific assignment and structure determination in protonless NMR experiments. J Magn Reson 182:325–329
Bax A (1989) Homonuclear magnetization transfer experiments using isotropic and nonisotropic mixing schemes. Israel J Chem 28:309–317
Bennett AE, Griffin RG, Ok JH, Vega S (1992) Chemical shift correlation spectroscopy in rotating solids: radio frequency-driven dipolar recoupling and longitudinal exchange. J Chem Phys 96:8624–8627
Bermel W, Bertini I, Felli IC, Kümmerle R, Pierattelli R (2003) 13C direct detection experiments on the paramagnetic oxidized monomeric copper, zinc superoxide dismutase. J Am Chem Soc 125:16423–16429
Bermel W, Bertini I, Duma L, Emsley L, Felli IC, Pierattelli R, Vasos PR (2005a) Complete assignment of heteronuclear protein resonances by protonless NMR spectroscopy. Angew Chem Int Ed 44:3089–3092
Bermel W, Bertini I, Felli IC, Pierattelli R, Vasos PR (2005b) A selective experiment for the sequential protein backbone assignment from 3D heteronuclear spectra. J Magn Reson 172:324–328
Bermel W, Bertini I, Felli IC, Kümmerle R, Pierattelli R (2006a) Novel 13C direct detection experiments, including extension to the third dimension, to perform the complete assignment of proteins. J Magn Reson 178:56–64
Bermel W, Bertini I, Felli IC, Lee Y-M, Luchinat C, Pierattelli R (2006b) Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J Am Chem Soc 128:3918–3919
Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R (2006c) 13C-detected protonless NMR spectroscopy of proteins in solution. Progr NMR Spectrosc 48:25–45
Bermel W, Felli IC, Matzapetakis M, Pierattelli R, Theil EC, Turano P (2007) A method for Cα direct-detection in protonless NMR. J Magn Reson 188:301–310
Bermel W, Bertini I, Csizmok V, Felli IC, Pierattelli R and Tompa P (accepted) H-start for exclusively heteronuclear NMR spectroscopy: the case of intrinsically disordered proteins
Bertini I, Lee Y-M, Luchinat C, Piccioli M, Poggi L (2001) Locating the metal ion in calcium-binding proteins by using cerium(III) as a probe. ChemBioChem 2:550–558
Bertini I, Duma L, Felli IC, Fey M, Luchinat C, Pierattelli R, Vasos PR (2004a) A heteronuclear direct detection NMR experiment for protein backbone assignment. Angew Chem Int Ed 43:2257–2259
Bertini I, Felli IC, Kümmerle R, Luchinat C, Pierattelli R (2004b) 13C–13C NOESY: a constructive use of 13C–13C spin-diffusion. J Biomol NMR 30:245–251
Bertini I, Felli IC, Kümmerle R, Moskau D, Pierattelli R (2004c) 13C–13C NOESY: an attractive alternative to study large macromolecules. J Am Chem Soc 126:464–465
Bertini I, Felli IC, Luchinat C, Parigi G, Pierattelli R (2007) Towards a protocol for solution structure determination of copper(II) proteins: the case of Cu(II)Zn(II) superoxide dismutase. ChemBioChem 8:1422–1429
Boehlen J-M, Bodenhausen G (1993) Experimental aspects of chirp NMR spectroscopy. J Magn Reson Ser A 102:293–301
Braunschweiler L, Ernst RR (1983) Coherence transfer by isotropic mixing: application to proton correlation spectroscopy. J Magn Reson 53:521–528
Breitmaier E, Jung G, Voelters W (1970) Pulse Fourier transform 13C-NMR spectroscopy, principles and applications. Angew Chem Int Ed Engl 10:673–686
Briand J, Ernst RR (2008) Computer-optimized homonuclear TOCSY experiments with suppression of cross relaxation. Chem Phys Lett 185:276
Caillet-Saguy C, Delepierre M, Lecroisey A, Bertini I, Piccioli M, Turano P (2006) Direct detected 13C NMR to investigate the Iron(III) hemophore HasA. J Am Chem Soc 128:150–158
Eaton HL, Fesik SW, Glaser SJ, Drobny GB (1990) Time dependence of 13C–13C magnetization transfer in isotropic mixing experiments involving amino acid spin systems. J Magn Reson 90:452–463
Eletsky A, Moreira O, Kovacs H, Pervushin K (2003) A novel strategy for the assignment of side-chain resonances in completely deuterated large proteins using (13)C spectroscopy. J Biomol NMR 26:167–179
Emsley L, Bodenhausen G (1990) Gaussian pulse cascades: new analytical functions for rectangular selective inversion and in-phase excitation in NMR. Chem Phys Lett 165:469–476
Farès C, Amata I, Carlomagno T (2007) 13C-detection in RNA bases: revealing structure–chemical shift relationships. J Am Chem Soc 129:15814–15823
Fiala R, Sklenar V (2007) 13C-detected NMR experiments for measuring chemical shifts and coupling constants in nucleic acid bases. J Biomol NMR 39:152–163
Furrer J, Kramer F, Marino JP, Glaser SJ, Luy B (2004) Homonuclear Hartmann–Hahn transfer with reduced relaxation losses by use of the MOCCA-XY16 multiple pulse seqeunce. J Magn Reson 166:39–46
Glaser SJ, Drobny GP (1990) Assessment and optimization of pulse sequences for homonuclear isotropic mixing. In: Warren WS (ed) Advances in magnetic resonance, vol 14. Academic Press, New York, pp 35–58
Glaser SJ, Quant JJ (1996) Homonuclear and heteronuclear Hartmann–Hahn transfer in isotropic liquids. In: Warren WS (ed) Advances in magnetic and optical resonance, vol 19. Academic Press, New York, pp 59–252
Griesinger C, Ernst RR (1988) Cross relaxation in time dependent nuclear spin systems—invariant trajectory approach. Chem Phys Lett 152:239–247
Grzesiek S, Bax A (1997) A three-dimensional NMR experiment with improved sensitivity for carbonyl–carbonyl J correlation in proteins. J Biomol NMR 9:207–211
Gullion T, Baker DB, Conradi MS (1990) New, compensated Carr–Purcell sequences. J Magn Reson 89:479–484
Hu K, Vögeli B, Clore GM (2007) Spin-state selective carbon-detected HNCO with TROSY optimization in all dimensions and double echo–antiecho sensitivity enhancement in both indirect dimensions. J Am Chem Soc 129:5484–5491
Kadkhodaie M, Rivas O, Tan M, Mohebbi A, Shaka AJ (1991) Broadband homonuclear cross polarization using flip–flop spectroscopy. J Magn Reson 91:437–443
Klages J, Kessler H, Glaser SJ, Luy B (2007) J-ONLY-TOCSY: efficient suppression of RDC-induced transfer in homonuclear TOCSY experiments using JESTER-1-derived multiple pulse sequences. J Magn Reson 189:217–227
Kolczak U, Salgado J, Siegal G, Saraste M, Canters GW (1999) Paramagnetic NMR studies of blue and purple copper proteins. Biospectroscopy 5:S19–S32
Kostic M, Pochapsky SS, Pochapsky TC (2002) Rapid recycle 13C, 15N and 13C,13C heteronuclear and homonuclear multiple quantum coherence detection for resonance assignments in paramagnetic proteins: example of Ni2+-containing acireductone dioxygenase. J Am Chem Soc 124:9054–9055
Kovacs H, Moskau D, Spraul M (2005) Cryogenically cooled probes—a leap in NMR technology. Progr NMR Spectrosc 46:131–155
Kramer F, Peti W, Griesinger C, Glaser SJ (2001) Optimized homonuclear Carr–Purcell-type dipolar mixing sequences. J Magn Reson 149:58–66
Liu A, Riek R, Wider G, Von Schroetter C, Zahn R, Wüthrich K (2000) NMR experiments for resonance assignments of 13C, 15N doubly-labeled flexible polypeptides: application to the human prion protein hPrP(23-230). J Biomol NMR 16:127–138
Lizak MJ, Gullion T, Conradi MS (1991) Measurements of like-spin dipole couplings. J Magn Reson 91:254–260
Luy B, Glaser SJ (2001) Superposition of scalar and residual dipolar couplings: analytical transfer functions for three spins 1/2 under cylindrical mixing conditions. J Magn Reson 148:169–181
Luy B, Schedletzky O, Glaser SJ (1999) Analytical polarization transfer functions for four coupled spins 12 under isotropic mixing conditions. J Magn Reson 138:19–27
Machonkin TE, Westler WM, Markley JL (2002) 13C–13C 2D NMR: a novel strategy for the study of paramagnetic proteins with slow electronic relaxation times. J Am Chem Soc 124:3204–3205
Mandel AM, Akke M, Palmer AG (1996) Dynamics of ribonuclease H: temperature dependence of motions on multiple time scales. Biochemistry 35:16009–16023
Matzapetakis M, Turano P, Theil EC, Bertini I (2007) 13C–13C NOESY spectra of a 480 kDa protein: solution NMR of ferritin. J Biomol NMR 38:237–242
Mulder FAA, Skrynnikov NR, Hon B, Dahlquist FW, Kay LE (2001) Measurement of slow (μs–ms) time scale dynamics in protein side chains by N-15 relaxation dispersion NMR spectroscopy: application to Asn and Gln residues in a cavity mutant of T4 lysozyme. J Am Chem Soc 123:967–975
Oh B-H, Westler WM, Darba P, Markley JL (1988) Protein carbon-13 spin systems by a single two-dimensional nuclear magnetic resonance experiment. Science 240:908–911
Palmer AGIII, Skelton NJ, Chazin WJ, Wright PE, Rance M (1992) Suppression of the effects of cross-correlation between dipolar and anisotropic chemical shift relaxation mechanisms in the measurement of spin–spin relaxation rates. Mol Phys 75:699–711
Schedletzky O, Glaser SJ (1996) Analytical coherence-transfer functions for the general AMX spin system under isotropic mixing. J Magn Reson Ser A 123:174–180
Shaka AJ, Keeler J, Freeman R (1983) Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson 53:313–340
Shaka AJ, Barker PB, Freeman R (1985) Evaluation of a new broadband decoupling sequence: WALTZ-16. J Magn Reson 64:547–552
Shaka AJ, Lee CJ, Pines A (1988) Iterative schemes for bilinear operators: application to spin decoupling. J Magn Reson 77:274–293
Skrynnikov NR, Mulder FAA, Hon B, Dahlquist FW, Kay LE (2001) Probing slow time scale dynamics at methyl-containing side chains in proteins by relaxation dispersion NMR measurements: application to methionine residues in a cavity mutant of T4 lysozyme. J Am Chem Soc 123:4556–4566
Sodickson DK, Levitt MH, Vega S, Griffin RG (1993) Broad-band dipolar recoupling in the nuclear magnetic resonance of rotating solids. J Chem Phys 98:6742–6748
Vincent SJF, Zwahlen C, Bodenhausen G (1996) Suppression of spin diffusion in selected frequency bands of nuclear Overhauser spectra. J Biomol NMR 7:169–172
Vögeli B, Kovacs H, Pervushin K (2004) Measurements of side chain 13C–13C residual dipolar coupling in uniformly deuterated proteins. J Am Chem Soc 126:2414–2420
Acknowledgments
This work has been supported in part by the EC contracts EU-NMR n° 026145 (TA and JRA) and SPINE II n° 031220 and by Ente Cassa di Risparmio di Firenze. S. J. G. and B. L. thank the Fonds der Chemischen Industrie and the Deutsche Forschungsgemeinschaft for financial support (GL 203/6–1; Emmy Noether and Heisenberg fellowships LU 835/1–4 and LU 835/2–1). B. L. thanks the Center for Integrated Protein Science Munich (CIPSM) for financial support. Prof. Ivano Bertini is gratefully acknowledged for stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Felli, I.C., Pierattelli, R., Glaser, S.J. et al. Relaxation-optimised Hartmann–Hahn transfer using a specifically Tailored MOCCA-XY16 mixing sequence for carbonyl–carbonyl correlation spectroscopy in 13C direct detection NMR experiments. J Biomol NMR 43, 187–196 (2009). https://doi.org/10.1007/s10858-009-9302-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-009-9302-6