Abstract
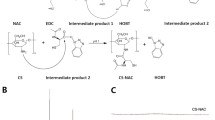
The ocular bioavailability of lipophilic drugs, such as dexamethasone, depends on both drug water solubility and mucoadhesion/permeation. Cyclodextrins and chitosan are frequently employed to either improve drug solubility or prolong drug contact onto mucosae, respectively. Although the covalent conjugation of cyclodextrin and chitosan brings to mucoadhesive drug complexes, their water solubility is restricted to acidic pHs. This paper describes a straightforward grafting of methyl-β-cyclodextrin (MCD) on quaternary ammonium chitosan (QA-Ch60), mediated by hexamethylene diisocyanate. The resulting product is a water-soluble chitosan derivative, having a 10-atom long spacer between the quaternized chitosan and the cyclodextrin. The derivative is capable of complexing the model drug dexamethasone and stable complexes were also observed for the lyophilized products. Furthermore, the conjugate preserves the mucoadhesive properties typical of quaternized chitosan and its safety as solubilizing excipient for ophthalmic applications was preliminary assessed by in vitro cytotoxicity evaluations. Taken as a whole, the observed features appear promising for future processing of the developed product into 3D solid forms, such as controlled drug delivery systems, films or drug eluting medical devices.







Similar content being viewed by others
References
Liu L, Zhu S. A study on the supramolecular structure of inclusion complex of β-cyclodextrin with prazosin hydrochloride. Carbohydr Polym. 2007;68:472–6. https://doi.org/10.1016/j.carbpol.2006.11.007.
Marques HMC, Hadgraft J, Kellaway IW. Studies of cyclodextrin inclusion complexes. I. The salbutamol-cyclodextrin complex as studied by phase solubility and DSC. Int J Pharm. 1990;63:259–66. https://doi.org/10.1016/0378-5173(90)90132-N.
Yang JS, Yang L. Preparation and application of cyclodextrin immobilized polysaccharides. J Mater Chem B. 2013;1:909–18. https://doi.org/10.1039/c2tb00107a.
Hansch C, Lao A, Hoekman D. Exploring QSAR. Hydrophobic, electronic and steric constants. Crit Rev Toxicol. 1995;25:67–89.
Rodríguez Villanueva J, Rodríguez Villanueva L, Guzmán Navarro M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int J Pharm. 2017;516:342–51.
Loftsson T, Stefánsson E Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int J Pharmaceut. 2017. https://doi.org/10.1016/j.ijpharm.2017.04.010.
Di Colo G, Zambito Y, Zaino C, Sansò M. Selected polysaccharides at comparison for their mucoadhesiveness and effect on precorneal residence of different drugs in the rabbit model. Drug Dev Ind Pharm. 2009;35:941–9.
Fabiano A, Chetoni P, Zambito Y. Mucoadhesive nano-sized supramolecular assemblies for improved pre-corneal drug residence time. Drug Dev Ind Pharm. 2015;41:2069–76.
Piras AM, Sandreschi S, Maisetta G, Esin S, Batoni G, Chiellini F. Chitosan nanoparticles for the linear release of model cationic peptide. Pharm Res. 2015;32:2259–65. https://doi.org/10.1007/s11095-014-1615-9.
Dash M, Samal SK, Douglas TEL, Schaubroeck D, Leeuwenburgh SC, Van Der Voort P, et al. Enzymatically biomineralized chitosan scaffolds for tissue-engineering applications. J Tissue Eng Regen Med. 2017;11:1500–13. https://doi.org/10.1002/term.2048
Piras AM, Maisetta G, Sandreschi S, Gazzarri M, Bartoli C, Grassi L, et al. Chitosan nanoparticles loaded with the antimicrobial peptide temporin B exert a long-term antibacterial activity in vitro against clinical isolates of Staphylococcus epidermidis. Front Microbiol. 2015;6(APR). https://doi.org/10.3389/fmicb.2015.00372.
Henriksen I, Green KL, Smart JD, Smistad G, Karlsen J. Bioadhesion of hydrated chitosans: an in vitro and in vivo study. Int J Pharm. 1996;145:231–40. https://doi.org/10.1016/S0378-5173(96)04776-X.
Lehr C-M, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–8. https://doi.org/10.1016/0378-5173(92)90353-4.
Tamer TM, Hassan MA, Omer AM, Valachova K, Eldin MSM, Collins MN. et al. Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr Polym. 2017;169:441–50. https://doi.org/10.1016/j.carbpol.2017.04.027.
Prabaharan M, Mano JF. Chitosan derivatives bearing cyclodextrin cavitiesas novel adsorbent matrices. Carbohydr Polym. 2006;63:153–66. https://doi.org/10.1016/j.carbpol.2005.08.051.
Chaleawlert-umpon S, Nuchuchua O, Saesoo S, Gonil P, Ruktanonchai UR, Sajomsang W. et al. Effect of citrate spacer on mucoadhesive properties of a novel water-soluble cationic β-cyclodextrin-conjugated chitosan. Carbohydr Polym. 2011;84:186–94. https://doi.org/10.1016/j.carbpol.2010.11.017.
Laine V, Coste-Sarguet A, Gadelle A, Defaye J, Perly B, Djedaini-Pilard F. Inclusion and solubilization properties of 6-S-glycosyl-6-thio derivatives of [small beta]-cyclodextrin. J Chem Soc, Perkin Trans. 1995;2:1479–87. https://doi.org/10.1039/P29950001479.
Zambito Y, Felice F, Fabiano A, Di Stefano R, Di Colo G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr Polym. 2013;92:33–9. https://doi.org/10.1016/j.carbpol.2012.09.029.
Zambito Y, Uccello-Barretta G, Zaino C, Balzano F, Di Colo G. Novel transmucosal absorption enhancers obtained by aminoalkylation of chitosan. Eur J Pharm Sci. 2006;29:460–9. https://doi.org/10.1016/j.ejps.2006.09.001.
Zambito Y, Zaino C, Uccello Barretta G, Balzano F, Di Colo G. Improved synthesis of quaternary ammonium–chitosan conjugates (N+–Ch) for enhanced intestinal drug permeation. Eur J Pharm Sci. 2008;33:343–50.
Jansook P, Loftsson T. CDs as solubilizers: effects of excipients and competing drugs. Int J Pharm. 2009;379:32–40.
Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–66.
Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212.
Hassan E, Gallo J. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–5.
Chetoni P, Monti D, Tampucci S, Matteoli B, Ceccherini-Nelli L, Subissi A, et al. Liposomes as a potential ocular delivery system of distamycin A. International. J Pharm. 2015;492:120–6.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–21.
Gonil P, Sajomsang W, Ruktanonchai UR, Pimpha N, Sramala I, Nuchuchua O. et al. Novel quaternized chitosan containing β-cyclodextrin moiety: synthesis, characterization and antimicrobial activity. Carbohydr Polym. 2011;83:905–13. https://doi.org/10.1016/j.carbpol.2010.08.080.
Song LX, Teng CF, Xu P, Wang HM, Zhang ZQ, Liu QQ. Thermal decomposition behaviors of β-cyclodextrin, its inclusion complexes of alkyl amines, and complexed β-cyclodextrin at different heating rates. J Incl Phenom Macrocycl Chem. 2007;60:223–33. https://doi.org/10.1007/s10847-007-9369-1.
Burgalassi S, Nicosia N, Monti D, Falcone G, Boldrini E, Chetoni P. Larch arabinogalactan for dry eye protection and treatment of corneal lesions: investigations in rabbits. J Ocul Pharmacol Ther. 2007;23:541–50.
Rassu G, Gavini E, Jonassen H, Zambito Y, Fogli S, Breschi MC. et al. New chitosan derivatives for the preparation of rokitamycin loaded microspheres designed for ocular or nasal administration. J Pharm Sci. 2009;98:4852–65. https://doi.org/10.1002/jps.21751.
Uccello-Barretta G, Balzano F, Aiello F, Senatore A, Fabiano A, Zambito Y. Mucoadhesivity and release properties of quaternary ammonium-chitosan conjugates and their nanoparticulate supramolecular aggregates: an NMR investigation. Int J Pharm. 2014;461:489–94. https://doi.org/10.1016/j.ijpharm.2013.12.018.
Kono H, Teshirogi T. Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int J Biol Macromol. 2015;72:299–308.
Wilson LD, Pratt DY, Kozinski JA. Preparation and sorption studies of β-cyclodextrin–chitosan–glutaraldehyde terpolymers. J Colloid Interface Sci. 2013;393:271–7. https://doi.org/10.1016/j.jcis.2012.10.046.
Yuan Z, Yeb Y, Gao F, Yuan H, Lan M, Lou K, et al. Chitosan-graft-b-cyclodextrin nanoparticles as a carrier for controlled drug release. Int J Pharm. 2013;446:191–8.
Sajomsang W, Nuchuchua O, Saesoo S, Gonil P, Chaleawlert-umpon S, Pimpha N. et al. A comparison of spacer on water-soluble cyclodextrin grafted chitosan inclusion complex as carrier of eugenol to mucosae. Carbohydr Polym. 2013;92:321–7. https://doi.org/10.1016/j.carbpol.2012.08.106.
Concheiro A, Alvarez-Lorenzo C. Chemically cross-linked and grafted cyclodextrin hydrogels: from nanostructures to drug-eluting medical devices. Adv Drug Deliv Rev. 2013;65:1188–203.
Salmaso S, Semenzato A, Bersani S, Matricardi P, Rossi F, Caliceti P. Cyclodextrin/PEG based hydrogels for multi-drug delivery. Int J Pharm. 2007;345:42–50. https://doi.org/10.1016/j.ijpharm.2007.05.035.
Ma B, Lee W-C. A modified Curtius reaction: an efficient and simple method for direct isolation of free amine. Tetrahedron Lett. 2010;51:385–6.
Liu X-M, Lee H-T, Reinhardt RA, Marky LA, Wang D. Novel biomineral-binding cyclodextrins for controlled drug delivery in the oral cavity. J Control Release. 2007;122:54–62.
Magnusdottir A, Másson M, Loftsson T. Self association and cyclodextrin solubilization of NSAIDs. J Incl Phenom. 2002;44:213–8. https://doi.org/101023/A:1023079322024.
Vianna RFL, Bentley MVLB, Ribeiro G, Carvalho FS, Neto AF, de Oliveira DCR, et al. Formation of cyclodextrin inclusion complexes with corticosteroids. Int J Pharm. 1998;167:205–13.
Buranaboripan W, Lang W, Motomura E, Sakairi N. Preparation and characterization of polymeric host molecules,b-cyclodextrin linked chitosan derivatives having different linkers. Int J Biol Macromol. 2014;69:27–34.
Mateen R, Hoare T. Carboxymethyl and hydrazide functionalized beta-cyclodextrin derivatives: a systematic investigation of complexation behaviours with the model hydrophobic drug dexamethasone. Int J Pharm. 2014;472:315–26. https://doi.org/10.1016/j.ijpharm.2014.06.046.
Simões SMN, Rey-Rico A, Concheiro A, Alvarez-Lorenzo C. Supramolecular cyclodextrin-based drug nanocarriers. Chem Commun. 2015;51:6275–89. https://doi.org/10.1039/c4cc10388b.
Acknowledgements
We gratefully acknowledge the financial support from P.R.A. 2017 by the University of Pisa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Piras, A.M., Zambito, Y., Burgalassi, S. et al. A water-soluble, mucoadhesive quaternary ammonium chitosan-methyl-β-cyclodextrin conjugate forming inclusion complexes with dexamethasone. J Mater Sci: Mater Med 29, 42 (2018). https://doi.org/10.1007/s10856-018-6048-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-018-6048-2