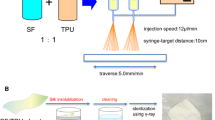
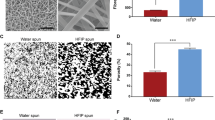
Abstract
Life-threatening cardiovascular anomalies require surgery for structural repair with cardiovascular patches. The biomaterial patch, derived from Bombyx mori silk fibroin (SF), is used as an alternative material due to its excellent tissue affinity and biocompatibility. However, SF lacks the elastomeric characteristics required for a cardiovascular patch. In order to overcome this shortcoming, we combined the thermoplastic polyurethane, Pellethane® (PU) with SF to develop an elastic biocompatible patch. Therefore, the purpose of this study was to investigate the feasibility of the blended SF/PU patch in a vascular model. Additionally, we focused on the effects of different SF concentrations in the SF/PU patch on its biological and physical properties. Three patches of different compositions (SF, SF7PU3 and SF4PU6) were created using an electrospinning method. Each patch type (n = 18) was implanted into rat abdominal aorta and histopathology was assessed at 1, 3, and 6 months post-implantation. The results showed that with increasing SF content the tensile strength and elasticity decreased. Histological evaluation revealed that inflammation gradually decreased in the SF7PU3 and SF patches throughout the study period. At 6 months post-implantation, the SF7PU3 patch demonstrated progressive remodeling, including significantly higher tissue infiltration, elastogenesis and endothelialization compared with SF4PU6. In conclusion, an increase of SF concentration in the SF/PU patch had effects on vascular remodeling and physical properties. Moreover, our blended patch might be an attractive alternative material that could induce the growth of a neo-artery composed of tissue present in native artery.
Graphical abstract








Similar content being viewed by others
References
Kazuro L, Fujimoto MD, Jianjun G, Hideki O, Tetsuro S, William RW. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg. 2007;83:648–54.
Igor T, Sava K, Tanja M, Omke T, Christoph B, Andres H, Axel H, Serghei C. Viable vascularized autologous patch for transmural myocardial reconstruction. Eur J Cardio Thorac Surg. 2009;36:306–11.
David K, Emile B. New Technologies for surgery of the congenital cardiac defect. Rambam Maimonides Med J. 2013;4:1–14.
Seokwon P, Jeffrey GJ. Biomaterials advances in patches for congenital heart defect repair. J. of Cardiovasc. Trans. Res. 2011;4:646–54.
Sakai T, Li RK, Weisel RD, Mickle DA, Kim ETJ, Jia ZQ, Yau TM. The fate of a tissue engineered cardiac graft in the right ventricular outflow tract of the rat. J Thorac Cardiovasc Surg. 2001;121:932–42.
Charu V, David LK. Silk as biomaterial. Prog. Polym. Sci. 2007;32:991–1007.
Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–16.
Banani K, Rangam R, Subhas CK, Xungai W. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65:457–70.
Meinel L, Kaplan D. Silk constructs for delivery of musculoskeletal therapeutics. Adv Drug Deliv Rev. 2012;12:1111–22.
Omenetto FG, Kaplan DL. New opportunities for ancient material. Science. 2010;329:528–31.
Yang M, Yamauchi K, Kurokawa M, Asakura T. Design of silk-like biomaterials inspired by mussel-adhesive protein. Tissue Eng. 2007;13:2941–7.
Higa K, Takeshima N, Moro F, Kawakita T, Kawashima M, Demura M, Shimazaki J, Asakura T, Tsubota K, Shimmura S. Porous silk fibroin film as a transparent carrier for cultivated corneal epithelial sheets. J Biomater Sci Polym Ed. 2011;22:2261–76.
Nagano A, Tanioka Y, Sakurai N, Sezutsu H, Kuboyama N, Kiba H, Tanimoto Y, Nishiyama N, Asakura T. Regeneration of the femoral epicondyle on calcium-binding silk scaffolds developed using transgenic silk fibroin produced by transgenic silkworm. Acta Biomater. 2011;7:1192–201.
Zhang X, Baughman CB, Kaplan DL. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 2008;29:2217–27.
Handschel J, Meyer T, Wiesmann HP. Fundamentals of tissue engineering and regenerative medicine. New York: Springer; 2009.
Huang FH, Sun LZ, Zheng J. In vitro and in vivo characterization of a silk fibroin-coated polyester vascular prosthesis. Artif Organs. 2008;32:932–41.
The Lubrizol Corporation (2017). Pellethane® TPU. (online) https://www.lubrizol.com/en/Life-Sciences/Products?Prllethane-TPU (Accessed 27 Aug 2017).
Pinchuk L. A review of the biostability and carcinogenicity of polyurethanes in medicine and the new generation of biostable polyurethanes. J Biomater Sci Polymer Edn. 1994;6:225–67.
Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16.
Stokes K, Mc Venes R, Anderson JM. Polyurethane elastomer biostability. J Biomater Appl. 1995;9(4):321–54.
Jimenez G, Asai S, Shishido A, Sumita M. Effect of the soft segment on the fatique behavior of segmented polyurethanes. Eur Polym J. 2000;36(9):2039–50.
Chen JH, Wei J, Chang C, Laiw RF, Lee YD. Studies on segmented polyurethane for biomedical application: Effects of composition and hard-segment content on biocompatibility. J Biomed Mater Res A. 1998;41(4):633–48.
Bergmeister H, Schreiber C, Grasl C, Walter I, Plasenzotti R, Stoiber M, Schima H. Healing characteristics of electrospun polyurethane grafts with various porosities. Acta Biomater. 2013;9(4):6032–40.
Chiarini A, Petrini P, Bozzini S, Pra ID, Armato U. Silk fibroin/poly(carbonate)-urethane as a substrate for cell growth: in vitro interactions with human cells. Biomaterials. 2003;24:789–99.
Park HS, Gong MS, Park JH, Moon SI, Wall IB, Kim HW, Lee JH, Jonathan CK. Silk fibroin–polyurethane blends: Physical properties and effect of silk fibroin content on viscoelasticity, biocompatibility and myoblast differentiation. Acta Biomater. 2013;9:8962–71.
Scott SM, Gaddy LR, Sahmel R, Hoffman H. A collagen coated vascular prosthesis. J Cardiovasc Surg. 1987;28:498–504.
Fukayama T, Takagi K, Tanaka R, Hatakeyama Y, Aytemiz D, Suzuki Y, Asakura T. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. Ann Vasc Surg. 2015;29:341–52.
Soldani G, Losi P, Bernabei M, Burchielli S, Chiappino D, Kull S, Briganti E, Spiller D. Long term performance of small-diameter vascular grafts made of a poly(ether)urethane-polydimethylsiloxane semiinterpenetrating polymeric network. Biomaterials. 2010;31:2592–605.
Wu HC, Wang TW, Kang PL, Tsuang YH, Sun JS, Lin FH. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. Biomaterials. 2007;28:1385–92.
Ahmed M, Ramos TA, Damanik F, Le BQ, Wieringa P, Bennink M, Blitterswijk CV, Boer J, Moroni L. A combinatorial approach towards the design of nanofibrous scaffolds for chondrogenesis. Sci Rep. 2014;5:14804.
Assoul N, Flaud P, Chaouat M, Letourneur D, Bataille I. Mechanical properties of rat thoracic and abdominal aortas. J Biomech. 2008;41(10):2227–36.
Akhtar R, Sherratt MJ, Cruickshank JK, Derby B. Characterizing the elastic properties of tissue. Mater Today. 2011;14(3):96–105.
Abé H, Hayashi K. Data book on mechanical properties of living cells, tissues, and organs. Tokyo: Springer; 1996.
García-Herrera CM, Atienza JM, Rojo FJ, Claes E, Guinea GV, Celentano DJ, Burgos RL. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med Biol Eng Comput. 2012;50(6):559–66.
Renna NF, de las Heras N, Miatello RM. Pathophysiology of vascular remodeling in hypertension. Int J Hyper. 2013;2013:1–7.
Lamba NM, Woodhouse KA, Cooper SL. Polyurethanes Biomed Appl. CRC Press;1997. pp. 132–135.
Rhodes NP, Bellón JM, Buján MJ, Soldani G, Hunt JA. Inflammatory response to a novel series of siloxane-crosslinked polyurethane elastomers having controlled biodegradation. J Mater Sci Mater Med. 2005;16:1207–11.
Lemson MS, Tordoir JHM, Daemen MJAP, Kitslaar PJEHM. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg. 2000;19:336–50.
Fukayama T, Ozai Y, Shimokawadoko H, Aytemiz D, Tanaka R, Machida N, Asakura T. Effect of fibroin sponge coating on in vivo performance of knitted silk small diameter vascular grafts. Organogenesis. 2015;11:137–151.
Ombrellaro MP, Stevens SL, Sciarrotta J, Freeman MB, Goldman MH. Effect of endoluminal PTFE graft placement on cell proliferation, PDGF secretion, and intimal hyperplasia. J Surg Res. 1996;63:110–114.
Fukunishi T, Best CA, Sugiura T, Shoji T, Yi T, Udelsman B, Ohst D, Ong CS, Zhang H, Shinoka T, Breuer CK, Johnson J, Hibino N. Tissue-Engineered Small Diameter Arterial Vascular Grafts from Cell-Free Nanofiber PCL/Chitosan Scaffolds in a Sheep Model. PloS ONE. 2016;11:e0158555.
Danenberg HD, Welt FG, Walker M, Seifert P, Toegel GS, Edelman ER. Systemic inflammation induced by lipopolysaccharide increases neointimal formation after balloon and stent injury in rabbits. Circulation. 2002;105:2917–22.
Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71:40–9.
Faury G. Function–structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol Biol. 2001;49:310–25.
Long JL, Tranquillo RT. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339–50.
Anidjar S, Dobrin PB, Eichorst M, Graham GP, Chejfec G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J Vasc Surg. 1992;16:139–47.
ZENA Werb, Banda, Jones MJ. PA. Degradation of connective tissue matrices by macrophages. J Exp Med. 1980;152:1537–53.
Pearson JD. Endothelial cell function and thrombosis. Bailieres Best Pract Res Clin Haematol. 1999;12:329–41.
Narayan D, Venkatraman SS. Effect of pore size and interpore distance on endothelial cell growth on polymers. J Biomed Mater Res A. 2008;87:710–8.
Noishiki Y, Tomizawa Y, Yamane Y, Matsumoto A. Autocrine angiogenic vascular prosthesis with bone marrow transplantation. Nat Med. 1996;2:90–3.
Khan OF, Sefton MV. Endothelialized biomaterials for tissue engineering applications in vivo. Trend Biotechnol. 2011;29:379–87.
Hayabuchi Y, Mori K, Kitagawa T, Sakata M, Kagami S. Polytetrafluoroethylene graft calcification in patients with surgically repaired congenital heart disease: evaluation using multidetector-row computed tomography. Am Heart J. 2007;153(806):e1–e8.
Park JC, Song MJ, Hwang YS, Suh H. Calcification comparison of polymers for vascular graft. Yonsei Med J. 2001;42:304–10.
Soldani G, Panol G, Sasken HF, Goddard MB, Galletti PM. Small diameter polyurethane-polydimethylsiloxane vascular prostheses made by a spraying, phase-inversion process. J Mater Sci Mater Med. 1992;3:106–13.
Acknowledgements
The authors would like to thank Professor Dr. Noboru Machida and Professor Dr. Tetsuo Asakura for providing us with the histological facility and the imaging analysis system. We are also grateful to Dr. Tsunenori Kameda and Dr. Taiyo Yoshioka for providing us with the tensile testing machine. This project is entrusted to us by MAFF as part of Scientific technique research promotion program of agriculture, forestry, fishers and food industry (26051A). And, some of achievements were result from supports of grant, KAKENHI, from Grant-in-Aid for Scientific Research (15H03020) by Ministry of Education, Culture, Sports, Science and Technology Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chantawong, P., Tanaka, T., Uemura, A. et al. Silk fibroin-Pellethane® cardiovascular patches: Effect of silk fibroin concentration on vascular remodeling in rat model. J Mater Sci: Mater Med 28, 191 (2017). https://doi.org/10.1007/s10856-017-5999-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5999-z