Abstract
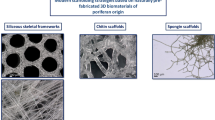
Gonads of sea urchin are consumed in Japan and some countries as food and most parts including its tests are discarded as marine wastes. Therefore, utilization of them as functional materials would reduce the waste as well as encourage Japanese fishery. In this study, magnesium containing calcite granules collected from sea urchin tests were hydrothermally phosphatized and the obtained granules were identified as approximately 82% in mass of magnesium containing β-tricalcium phosphate and 18% in mass of nonstoichiometric hydroxyapatite, i.e., a biphasic calcium phosphate, maintaining the original porous network. Shape-controlled scaffolds were fabricated with the obtained biphasic calcium phosphate granules and collagen. The scaffolds showed good open porosity (83.84%) and adequate mechanical properties for handling during cell culture and subsequent operations. The MG-63 cells showed higher proliferation and osteogenic differentiation in comparison to a control material, the collagen sponge with the same size. Furthermore, cell viability assay proved that the scaffolds were not cytotoxic. These results suggest that scaffold prepared using sea urchin test derived calcium phosphate and collagen could be a potential candidate of bone void fillers for non-load bearing defects in bone reconstruction as well as scaffolds for bone tissue engineering.










Similar content being viewed by others
References
Boskey AL, Coleman R. Aging and bone. J Dent Res United States. 2010;89:1333–48.
Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int Engl. 2003;14 Suppl 3:S35–42.
Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices Engl. 2006;3:49–57.
Finkemeier CG, Bone-grafting and bone-graft substitutes. J Bone Jt Surg Am. 2002;84−A:454–64
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:2.
Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng Annu Rev. 1999;1:19–46.
Fröhlich M, Grayson W, Wan L, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue Engineered Bone Grafts: Biological Requirements, Tissue Culture and Clinical Relevance. Curr. Stem Cell Res. Ther. 2008;3(4):254–264.
Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70.
Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91.
Oonishi H, Hench LL, Wilson J, Sugihara F, Tsuji E, Kushitani S, et al. Comparative bone growth behavior in granules of bioceramic materials of various sizes. J Biomed Mater Res. 1999;44:31–43.
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: A review. Int J Polym Sci. 2011; https://doi.org/10.1155/2011/290602.
LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;395:81–98.
Yuan H, Yang Z, Li Y, Zhang X, De Bruijn JD, De Groot K. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med. 1998;9:723–6.
Roy DM, Linnehan SK. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature. 1974;247:220–2.
Ben-Nissan B. Natural bioceramics: from coral to bone and beyond. Curr Opin Solid State Mater Sci. 2003;7:283–8.
Barros AA, Aroso IM, Silva TH, Mano JF, Duarte AR, Reis RL. In vitro bioactivity studies of ceramic structures isolated from marine sponges. Biomed Mater. 2016;11:45004.
Ivankovic H, Gallego Ferrer G, Tkalcec E, Orlic S, Ivankovic M. Preparation of highly porous hydroxyapatite from cuttlefish bone. J Mater Sci Mater Med. 2009;20:1039–46.
Vecchio KS, Zhang X, Massie JB, Wang M, Kim CW. Conversion of sea urchin spines to Mg-substituted tricalcium phosphate for bone implants. Acta Biomater Engl. 2007;3:785–93.
Clarke SA, Walsh P. Marine organisms for bone repair and regeneration. In: Mallick K, editor. Bone Substituted Biomaterials. 2014. p. 294–318.
Holzapfel BM, Reichert JC, Schantz J-T, Gbureck U, Rackwitz L, Nöth U, et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev. 2013;65:581–603.
Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29:1177–88.
Bohner M. Silicon-substituted calcium phosphates—a critical view. Biomaterials. 2009;30:6403–6.
Habibovic P, Barralet JE. Bioinorganics and biomaterials: bone repair. Acta Biomater Engl. 2011;7:3013–26.
Kannan S, Rocha JHG, Agathopoulos S, Ferreira JMF. Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. Acta Biomater. 2007;3:243–9.
Holmes RE, Bucholz RW, Mooney V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. A histometric study. J Bone Jt Surg Am. 1986;68:904–11.
Vuola J, Taurio R, Goransson H, Asko-Seljavaara S. Compressive strength of calcium carbonate and hydroxyapatite implants after bone-marrow-induced osteogenesis. Biomaterials. 1998;19:223–7.
Das S, Mangwani N. Ocean acidification and marine microorganisms: responses and consequences. Oceanologia. 2015;57:349–61.
Akino M, Aso S, Kimura M. Effectiveness of biological filter media derived from sea urchin skeletons. Fish Sci. 2015;81:923–7.
Tamasan M, Ozyegin LS, Oktar FN, Simon V. Characterization of calcium phosphate powders originating from Phyllacanthus imperialis and Trochidae Infundibulum concavus marine shells. Mater Sci Eng C. 2013;33:2569–77.
Ağaoğullari D, Kel D, Gökçe H, Duman I, Öveçoğlu ML. Bioceramic Production from Sea Urchins. Acta Physica Polonica A. 2012;121:1–4.
Borzęcka-Prokop B, Wesełucha-Birczyńska A, Koszowska E. MicroRaman, PXRD, EDS and microscopic investigation of magnesium calcite biomineral phases. The case of sea urchin biominerals. J Mol Struct. 2007;828:80–90.
Chang SJ, Huang Y-T, Yang S-C, Kuo S-M, Lee M-W. In vitro properties of gellan gum sponge as the dental filling to maintain alveolar space. Carbohydr Polym. 2012;88:684–9.
Tsipursky SJ, Buseck PR. Structure of magnesian calcite from sea urchins. Am Mineral. 1993;78:775–81.
Nyquist RA, Kagel RO. PREFACE BT—Handbook of infrared and Raman spectra of inorganic compounds and organic salts. San Diego: Academic Press; 1971. p. vii.
Zhang X, Vecchio KS. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front Mater Sci. 2013;7:103–17.
Ishikawa K. Bone substitute fabrication based on dissolution-precipitation reactions. Materials. 2010;3:1138–55.
Kannan S, Ventura JM, Ferreira JMF. Aqueous precipitation method for the formation of Mg-stabilized β-tricalcium phosphate: an X-ray diffraction study. Ceram Int. 2007;33:637–41.
Reddy MM, Nancollas GH. The crystallization of calcium carbonate. J Cryst Growth. 1976;35:33–8.
Safronova TV, Putlyaev VI, Avramenko OA, Shekhirev MA, Veresov AG. Ca-deficient hydroxyapatite powder for producing tricalcium phosphate based ceramics. Glas Ceram. 2011;68:28–32.
Loh QL, Choong C, Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B. 2013;19:485–502
Kikuchi M, Koyama Y, Edamura K, Irie A. Synthesis of hydroxyapatite/collagen bone-like nanocomposite and its biological reactions. Adv Nanocomposites—Synth Charact Ind Appl. 2007;2:181–94.
Sotome S, Ae K, Okawa A, Ishizuki M, Morioka H, Matsumoto S, et al. Efficacy and safety of porous hydroxyapatite/type 1 collagen composite implantation for bone regeneration: a randomized controlled study. J Orthop Sci. 2016;21:373–80.
Yoshida T, Kikuchi M, Koyama Y, Takakuda K. Osteogenic activity of MG63 cells on bone-like hydroxyapatite/collagen nanocomposite sponges. J Mater Sci Mater Med. 2010;21:1263–72.
Matsuno T, Nakamura T, Kuremoto K, Notazawa S, Nakahara T, Hashimoto Y, et al. Development of beta-tricalcium phosphate/collagen sponge composite for bone regeneration. Dent Mater J Jpn. 2006;25:138–44.
Lobo SE, Livingston Arinzeh T. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Mater. 2010;3:815–826.
Lee E-U, Kim D-J, Lim H-C, Lee J-S, Jung U-W, Choi S-H. Comparative evaluation of biphasic calcium phosphate and biphasic calcium phosphate collagen composite on osteoconductive potency in rabbit calvarial defect. Biomater Res. 2015;19:1–7.
Acknowledgements
The authors would like to thank Mr. Taira Sato for his help with the ICP-AES analysis. The authors express their sincere thanks to Prof. Yagi Hiroki, Otaru University of commerce, Japan and Higashi-shakotan fishery cooperative for providing sea urchin tests. This study in part was supported by revitalizing a local community by the development of new materials with sea urchin shells, the program for a proportion of practical uses with fish industries waste resources, Shakotan, Hokkaido, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Manchinasetty, N.V.L., Oshima, S. & Kikuchi, M. Preparation of flexible bone tissue scaffold utilizing sea urchin test and collagen. J Mater Sci: Mater Med 28, 184 (2017). https://doi.org/10.1007/s10856-017-5993-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5993-5