Abstract
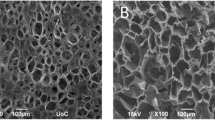
Chitosan scaffolds have gained much attention in various tissue engineering applications, but the effect of their microstructure on cell-material spatial interactions remains unclear. Our objective was to evaluate the effect of chitosan-based matrices doping with chitin nano-whiskers (CNW) on adhesion, spreading, cytoskeleton structure, and proliferation of rat bone marrow stromal cells (BMSCs). The behavior of BMSCs during culture on chitosan-CNW films was determined by the molecular mass, hydrophobicity, porosity, crosslinking degree, protonation degree and molecular structure of the composite chitosan-CNW films. The shape, spreading area, cytoskeleton structure, and proliferation of BMSCs on chitosan matrices with a crystalline structure and high porosity were similar to that observed for BMSCs cultured on polystyrene tissue culture plates. The amorphous polymer structure and high swelling led to a decrease in the spreading area and cell proliferation. Thus, we can control the behavior of cells in culture (adhesion, spreading, and proliferation) by changing the physico-chemical properties of the chitosan-CNW films.







Similar content being viewed by others
References
Lanza RP, Langer R, Vacanti JP. Principles of tissue engineering. 3rd ed. Academic Press; San Diego, CA, USA. 2011.
Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181(1):67–73. doi:10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C.
Gao J, Yao JQ, Caplan AI. Stem cells for tissue engineering of articular cartilage. Proc Inst Mech Eng H. 2007;221(5):441–50.
Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–92. doi:10.1634/stemcells.19-3-180.
Ciapetti G, Ambrosio L, Marletta G, Baldini N, Giunti A. Human bone marrow stromal cells: in vitro expansion and differentiation for bone engineering. Biomaterials. 2006;27(36):6150–60. doi:10.1016/j.biomaterials.2006.08.025.
Panarin EF, et al. Matrices for cell culture of human skin cells based on natural polysaccharides chitin and chitosan. Kletochnaya Transplantologiya i Tkanevaya Inzheneriya (Cell Transplantology and Tissue Engineering). 2009;4(3):42–6.
Shoichet MS. Polymer scaffolds for biomaterials applications. Macromolecules. 2010;43(2):581–91. doi:10.1021/ma901530r.
Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589–98.
Afanas’eva NV, et al. Molecular mobility of chitosan and its interaction with montmorillonite in composite films: dielectric spectroscopy and FTIR studies. Poly Sci Series A. 2013;55(12):738–48. doi:10.1134/S0965545X13120018.
Khan A, et al. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr Polym. 2012;90(4):1601–8. doi:10.1016/j.carbpol.2012.07.037.
Petrova VA, et al. Specific features of chitosan-montmorillonite interaction in an aqueous acid solution and properties of related composite films. Poly Sci Series A. 2012;54(3):224–30. doi:10.1134/S0965545X1203008X.
Shchipunov YA, Silant’ev VE, Postnova IV. Self-organization in the chitosan-clay nanoparticles system regulated through polysaccharide macromolecule charging. 1. Hydrogels. Colloid J. 2012;74(5):627–35. doi:10.1134/S1061933X12050092.
Fan Y, Saito T, Isogai A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr Polym. 2010;79(4):1046–51. doi:10.1016/j.carbpol.2009.10.044.
Kiroshka VV, et al. Adhesion, growth, and proliferation of endothelial cells on biopolymer extracellular film matrices. Bull Exp Biol Med. 2014;158(1):153–8. doi:10.1007/s10517-014-2712-9.
Zotkin MA, Vikhoreva GA, Kechek’yan AS. Thermal modification of chitosan films in the form of salts with various acids. Poly Sci Series B. 2004;46(1-2):39–42.
Nud’ga LA, et al. Chemical and structural transformations in chitosan films in the course of storage. Russ J Appl Chem. 2008;81(11):1992–6. doi:10.1134/S1070427208110244.
Pogodina NV, et al. Conformational characteristics of chitosan molecules as demonstrated by diffusion-sedimentation analysis and viscometry. Poly Sci USSR. 1986;28(2):251–9. doi:10.1016/0032-3950(86)90076-6.
Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009;10(7):1810–7. doi:10.1021/bm900240k.
Anokhina EB, Buravkova LB. Heterogeneity of stromal precursor cells isolated from rat bone marrow. Tsitologiia. 2007;49(1):40–7.
Huang Y, Siewe M, Madihally SV. Effect of spatial architecture on cellular colonization. Biotechnol Bioeng. 2006;93(1):64–75. doi:10.1002/bit.20703.
Lai JY, Lin PK, Hsiue GH, Cheng HY, Huang SJ, Li YT. Low bloom strength gelatin as a carrier for potential use in retinal sheet encapsulation and transplantation. Biomacromolecules. 2009;10(2):310–9. doi:10.1021/bm801039n.
Petrenko YA, Ivanov RV, Petrenko AY, Lozinsky VI. Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. J Mater Sci Mater Med. 2011;22(6):1529–40. doi:10.1007/s10856-011-4323-6.
Davis JM, ed. Basic cell culture. 2nd ed. Oxford University Press: Oxford, UK. 2002.
Yudin VE, et al. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr Polym. 2014;108:176–82. doi:10.1016/j.carbpol.2014.02.090.
Kulichikhin VG, Semakov AV, Karbushev VV, Platé NA, Picken SJ. The chaos-to-order transition in critical modes of shearing for polymer and nanocomposite melts. Poly Sci Series A. 2009;51(11):1303–12. doi:10.1134/S0965545X09110169.
Yin H, Mo D, Chen D. Orientation behavior of attapulgite nanoparticles in poly(acrylonitrile)/attapulgite solutions by rheological analysis. J Poly Sci Part B: Polym Phys. 2009;47(10):945–54. doi:10.1002/polb.21701.
Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–72. doi:10.1038/35074532.
Okuyama K, Noguchi K, Hanafusa Y, Osawa K, Ogawa K. Structural study of anhydrous tendon chitosan obtained via chitosan/acetic acid complex. Int J Biol Macromol. 1999;26(4):285–93.
Okuyama K, Noguchi K, Kanenari M, Egawa T, Osawa K, Ogawa K. Structural diversity of chitosan and its complexes. Carbohydr Poly. 2000;41(3):237–47. doi:10.1016/S0144-8617(99)00142-3.
Zhang Y, Xue C, Xue Y, Gao R, Zhang X. Determination of the degree of deacetylation of chitin and chitosan by X-ray powder diffraction. Carbohydr Res. 2005;340(11):1914–7. doi:10.1016/j.carres.2005.05.005.
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31(7):603–32. doi:10.1016/j.progpolymsci.2006.06.001.
Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24(24):4337–51.
Zaborowska M, Bodin A, Backdahl H, Popp J, Goldstein A, Gatenholm P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010;6(7):2540–7. doi:10.1016/j.actbio.2010.01.004.
Hillberg AL, Holmes CA, Tabrizian M. Effect of genipin cross-linking on the cellular adhesion properties of layer-by-layer assembled polyelectrolyte films. Biomaterials. 2009;30(27):4463–70. doi:10.1016/j.biomaterials.2009.05.026.
Mendelsohn JD, Yang SY, Hiller J, Hochbaum AI, Rubner MF. Rational design of cytophilic and cytophobic polyelectrolyte multilayer thin films. Biomacromolecules. 2003;4(1):96–106. doi:10.1021/bm0256101.
Acknowledgements
VA Petrova, DD Chernyakov, and YA Skorik are grateful to the Russian Science Foundation (project #16-19-10536) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kiroshka, V.V., Petrova, V.A., Chernyakov, D.D. et al. Influence of chitosan-chitin nanofiber composites on cytoskeleton structure and the proliferation of rat bone marrow stromal cells. J Mater Sci: Mater Med 28, 21 (2017). https://doi.org/10.1007/s10856-016-5822-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5822-2