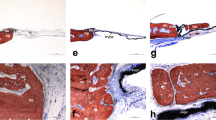
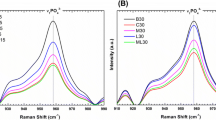
Abstract
Osteoporosis is a chronic disease that impairs proper bone remodeling. Guided bone regeneration is a surgical technique that improves bone defect in a particular region through new bone formation, using barrier materials (e.g. membranes) to protect the space adjacent to the bone defect. The polytetrafluorethylene membrane is widely used in guided bone regeneration, however, new membranes are being investigated. The purpose of this study was to evaluate the effect of P(VDFTrFE)/BT [poly(vinylidene fluoride-trifluoroethylene)/barium titanate] membrane on in vivo bone formation. Twenty-three Wistar rats were submitted to bilateral ovariectomy. Five animals were subjected to sham surgery. After 150 days, bone defects were created and filled with P(VDF-TrFE)/BT membrane or PTFE membrane (except for the sham and OVX groups). After 4 weeks, the animals were euthanized and calvaria samples were subjected to histomorphometric and computed microtomography analysis (microCT), besides real time polymerase chain reaction (real time PCR) to evaluate gene expression. The histomorphometric analysis showed that the animals that received the P(VDF-TrFE)/BT membrane presented morphometric parameters similar or even better compared to the animals that received the PTFE membrane. The comparison between groups showed that gene expression of RUNX2, BSP, OPN, OSX and RANKL were lower on P(VDF-TrFE)/BT membrane; the gene expression of ALP, OC, RANK and CTSK were similar and the gene expression of OPG, CALCR and MMP9 were higher when compared to PTFE. The results showed that the P(VDF-TrFE)/BT membrane favors bone formation, and therefore, may be considered a promising biomaterial to support bone repair in a situation of osteoporosis.




Similar content being viewed by others
References
International Osteoporosis Foundation. http://www.iofbonehealth.org/what-is-osteoporosis. Accessed 31 May 2016.
Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–8.
Siéssere S, de Albuquerque Lima N, Semprini M, de Sousa LG, Paulo Mardegan Issa J, Aparecida Caldeira, Monteiro S, Cecílio Hallak Regalo S. Masticatory process in individuals with maxillary and mandibular osteoporosis: electromyographic analysis. Osteoporos Int. 2009;20:1847–51.
Vishwanath SB, Kumar V, Kumar S, Shashikumar P, Shashikumar Y, Patel PV. Correlation of periodontal status and bone mineral density in postmenopausal women: a digital radiographic and quantitative ultrasound study. Indian J Dent Res. 2011;22:270–6.
Scalize PH, de Sousa LG, Regalo SC, Semprini M, Pitol DL, da Silva GA, de Almeida Coelho J, Coppi AA, Laad AA, Prado KF, Siessere S. Low-level laser therapy improves bone formation: stereology findings for osteoporosis in rat model. Lasers Med Sci. 2015;30:1599–607.
Ronda M, Rebaudi A, Torelli L, Stacchi C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: a prospective randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:859–66.
Dimitriou R, Mataliotakis GI, Calori GM, Giannoudis PV. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med. 2012;10:81
Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57:3–14.
Gimenes R, Zaghete MA, Bertolini M, Varela JA, Coelho LO, Silva NF Jr. Composites PVDF-TrFE/BT used as bioactive membranes for enhancing bone regeneration. In: Bar-Cohen Y, (ed.) Proceedings of SPIE: Smart Structures and Materials. Bellinghan, WA: SPIE; 2004;5385:539–47.
Beloti MM, de Oliveira PT, Gimenes R, Zaghete MA, Bertolini MJ, Rosa AL. In vitro biocompatibility of a novel membrane of the composite poly(vinylidene-trifluoroethylene)/barium titanate. J Biomed Mater Res A. 2006;79:282–8.
Teixeira LN, Crippa GE, Trabuco AC, Gimenes R, Zaghete MA, Palioto DB, de Oliveira PT, Rosa AL, Beloti MM. In vitro biocompatibility of poly(vinylidene fluoride-trifluoroethylene)/barium titanate composite using cultures of human periodontal ligament fibroblasts and keratinocytes. Acta Biomater. 2010;6:979–89.
Teixeira LN, Crippa GE, Gimenes R, Zaghete MA, de Oliveira PT, Rosa AL, Beloti MM. Response of human alveolar bone-derived cells to a novel poly(vinylidene fluoride-trifluoroethylene)/barium titanate membrane. J Mater Sci Mater Med. 2011;22:151–8.
Lopes HB, Santos TD, de Oliveira FS, Freitas GP, de Almeida AL, Gimenes R, Rosa AL, Beloti MM. Poly(vinylidene-trifluoroethylene)/barium titanate composite for in vivo support of bone formation. J Biomater Appl. 2014;29:104–12.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86.
Maniatopoulos C, Rodriguez A, Deporter DA, Melcher AH. An improved method for preparing histological sections of metallic implants. Int J Oral Maxillofac Implants. 1986;1:31–7.
Frost HM, Jee WS. On the rat model of human osteopenias and osteoporosis. Bone Miner. 1992;18:227–36.
Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:171–92.
Crump TB, Rivera-Hidalgo F, Harrison JW, Williams FE, Guo IY. Influence of three membrane types on healing of bone defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:365–74.
Salata LA, Hatton PV, Devlin AJ, Craig GT, Brook IM. In vitro and in vivo evaluation of e-PTFE and alkali-cellulose membranes for guided bone regeneration. Clin Oral Implants Res. 2001;12:62–8.
Amano Y, Ota M, Sekiguchi K, Shibukawa Y, Yamada S. Evaluation of a poly-l-lactic acid membrane and membrane fixing pin for guided tissue regeneration on bone defects in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:155–63.
Oortgiesen DA, Plachokova AS, Geenen C, Meijer GJ, Walboomers XF, van den Beucken JJ, Jansen JA. Alkaline phosphatase immobilization onto Bio-Gide® and Bio-Oss® for periodontal and bone regeneration. J Clin Periodontol. 2012;39:546–55.
Mardas N, Busetti J, de Figueiredo JA, Mezzomo LA, Scarparo RK, Donos N. Guided bone regeneration in osteoporotic conditions following treatment with zoledronic acid. Clin Oral Implants Res. 2016. doi:10.1111/clr.12810
van Houdt CI, Tim CR, Crovace MC, Zanotto ED, Peitl O, Ulrich DJ, Jansen JA, Parizotto NA, Renno AC, van den Beucken JJ. Bone regeneration and gene expression in bone defects under healthy and osteoporotic bone conditions using two commercially available bone graft substitutes. Biomed Mater. 2015. doi:10.1088/1748-6041/10/3/035003
Hirata HH, Munhoz MA, Plepis AM, Martins VC, Santos GR, Galdeano EA, Cunha MR. Feasibility study of collagen membranes derived from bovine pericardium and intestinal serosa for the repair of cranial defects in ovariectomized rats. Injury. 2015;46:1215–22.
Weber N, Lee YS, Shanmugasundaram S, Jaffe M, Arinzeh TL. Characterization and in vitro cytocompatibility of piezoelectric electrospun scaffolds. Acta Biomater. 2010;6:3550–6.
Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone. 1993;14:173–82.
Donate PB, Fornari TA, Macedo C, Cunha TM, Nascimento DC, Sakamoto-Hojo ET, Donadi EA, Cunha FQ, Passos GA. T cell post-transcriptional miRNA-mRNA interaction networks identify targets associated with susceptibility/resistance to collagen-induced arthritis. PLoS One. 2013;8:e54803
Kubo T, Shiga T, Hashimoto J, Yoshioka M, Honjo H, Urabe M, Kitajima I, Semba I, Hirasawa Y. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J Steroid Biochem Mol Biol. 1999;68:197–202.
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–6.
Torricelli P, Fini M, Giavaresi G, Giardino R. Human osteoblast cultures from osteoporotic and healthy bone: biochemical markers and cytokine expression in basal conditions and in response to 1.25(OH)2 D3. Art Cells Blood Subs Immob Biotech. 2002;30:219–27.
Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY, Yuan Y, Liu XZ, Bian JC, Liu ZP. Osteoprotegerin influences the bone resorption activity of osteoclasts. Int J Mol Med. 2013;31:1411–7.
Lima LL, Gonçalves PF, Sallum EA, Casati MZ, Nociti FH Jr Guided tissue regeneration may modulate gene expression in periodontal intrabony defects: a human study. J Periodontal Res. 2008;43:459–64.
Baxter FR, Bowen CR, Turner IG, Dent AC. Electrically active bioceramics: a review of interfacial responses. Ann Biomed Eng. 2010;38:2079–92.
Acknowledgments
Sebastião C. Bianco and Milla S. Tavares are acknowledged for technical assistance during the experiments.
Funding
This work was supported by São Paulo Research Foundation—FAPESP [grant number 2014/02984-0], National Council for Scientific and Technological [CNPq] and Coordination for the Improvement of Higher Education Personnel (CAPES). The synthesis of the PVDF-TrFE/BaTiO3 membranes was supported by Minas Gerais State Research Foundation (FAPEMIG, Brazil) under the Grant TEC - APQ-03013-15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Scalize, P.H., Bombonato-Prado, K.F., de Sousa, L.G. et al. Poly(Vinylidene Fluoride-Trifluorethylene)/barium titanate membrane promotes de novo bone formation and may modulate gene expression in osteoporotic rat model. J Mater Sci: Mater Med 27, 180 (2016). https://doi.org/10.1007/s10856-016-5799-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5799-x