Abstract
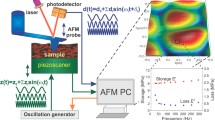
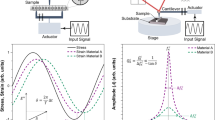
Scanning probe microscopy has been widely used to obtain topographical information and to quantify nanostructural properties of different materials. Qualitative and quantitative imaging is of particular interest to study material–material interactions and map surface properties on a nanoscale (i.e. stiffness and viscoelastic properties). These data are essential for the development of new biomedical materials. Currently, there are limited options to map viscoelastic properties of materials at nanoscale and at high resolutions. Lorentz contact resonance (LCR) is an emerging technique, which allows mapping viscoelasticity of samples with stiffness ranging from a few hundred Pa up to several GPa. Here we demonstrate the applicability of LCR to probe and map the viscoelasticity and stiffness of ‘soft’ (biological sample: cell treated with nanodiamond), ‘medium hard’ (pharmaceutical sample: pMDI canister) and ‘hard’ (human teeth enamel) specimens. The results allowed the identification of nanodiamond on the cells and the qualitative assessment of its distribution based on its nanomechanical properties. It also enabled mapping of the mechanical properties of the cell to demonstrate variability of these characteristics in a single cell. Qualitative imaging of an enamel sample demonstrated variations of stiffness across the specimen and precise identification of enamel prisms (higher stiffness) and enamel interrods (lower stiffness). Similarly, mapping of the pMDI canister wall showed that drug particles were adsorbed to the wall. These particles showed differences in stiffness at nanoscale, which suggested variations in surface composition—multiphasic material. LCR technique emerges as a valuable tool for probing viscoelasticity of samples of varying stiffness’s.





Similar content being viewed by others
References
Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986;56(9):930.
Haghi M, Traini D, Wood LG, Oliver B, Young PM, Chrzanowski W. A ‘soft spot’for drug transport: modulation of cell stiffness using fatty acids and its impact on drug transport in lung model. J Mater Chem B. 2015;3(13):2583–9.
Singh RK, Jin G-Z, Mahapatra C, Patel KD, Chrzanowski W, Kim H-W. Mesoporous Silica-Layered Biopolymer Hybrid Nanofibrous Scaffold: a Novel Nanobiomatrix Platform for Therapeutics Delivery and Bone Regeneration. ACS Appl Mater Interfaces. 2015;7(15):8088–98.
Hutmacher D, Chrzanowski W. Biointerfaces: Where Material Meets Biology. R Soc Chem. 2015.
Chen Q-Z, Ishii H, Thouas GA, Lyon AR, Wright JS, Blaker JJ, et al. An elastomeric patch derived from poly (glycerol sebacate) for delivery of embryonic stem cells to the heart. Biomaterials. 2010;31(14):3885–93.
Chrzanowski W, Neel EAA, Armitage DA, Knowles JC. Effect of surface treatment on the bioactivity of nickel–titanium. Acta Biomater. 2008;4(6):1969–84.
Chrzanowski W, Lee JH, Kondyurin A, Lord MS, Jang JH, Kim HW, et al. Nano-bio-chemical braille for cells: the regulation of stem cell responses using bi-functional surfaces. Adv Funct Mater. 2015;25(2):193–205.
Won J-E, Yun Y-R, Jang J-H, Yang S-H, Kim J-H, Chrzanowski W, et al. Multifunctional and stable bone mimic proteinaceous matrix for bone tissue engineering. Biomaterials. 2015;56:46–57.
Garcia R, Proksch R. Nanomechanical mapping of soft matter by bimodal force microscopy. Eur Polymer J. 2013;49(8):1897–906.
Proksch R, Kalinin S. Piezoresponse force microscopy with asylum research AFMs. PFM App Note. 2008.
Efimov AE, Saunin SA, Mescheryakov NA. Polymer elasticity analysis with atomic force acoustic microscopy. In: Arnold W, Hirsekorn S, editors. Acoustical imaging, vol 27. Netherlands: Springer; 2004. p. 729–33.
Yuya P, Hurley D, Turner JA. Contact-resonance atomic force microscopy for viscoelasticity. J Appl Phys. 2008;104(7):074916.
Stan G, King SW, Cook RF. Nanoscale mapping of contact stiffness and damping by contact resonance atomic force microscopy. Nanotechnology. 2012;23(21):215703.
Dillon E, Kjoller K, Prater C. Lorentz contact resonance imaging for atomic force microscopes: probing mechanical and thermal properties on the nanoscale. Microscopy Today. 2013;21(06):18–24.
Rabe U, Amelio S, Kopycinska M, Hirsekorn S, Kempf M, Göken M, et al. Imaging and measurement of local mechanical material properties by atomic force acoustic microscopy. Surf Interface Anal. 2002;33(2):65–70.
Lee B, Prater CB, King WP. Lorentz force actuation of a heated atomic force microscope cantilever. Nanotechnology. 2012;23(5):055709.
Buguin A, Du Roure O, Silberzan P. Active atomic force microscopy cantilevers for imaging in liquids. Appl Phys Lett. 2001;78(19):2982–4.
Marcott C, Lo M, Dillon E, Kjoller K, Prater C. Interface Analysis of Composites Using AFM-Based Nanoscale IR and Mechanical Spectroscopy. Microscopy Today. 2015;23(02):38–45.
Han W, Lindsay S, Jing T. A magnetically driven oscillating probe microscope for operation in liquids. Appl Phys Lett. 1996;69(26):4111–3.
Miyake K, Satomi N, Sasaki S. Elastic modulus of polystyrene film from near surface to bulk measured by nanoindentation using atomic force microscopy. Appl Phys Lett. 2006;89(3):031925.
Wang Z, Liang Z, Wang B, Zhang C, Kramer L. Processing and property investigation of single-walled carbon nanotube (SWNT) buckypaper/epoxy resin matrix nanocomposites. Compos A Appl Sci Manuf. 2004;35(10):1225–32.
Baidakova M, Kukushkina YA, Sitnikova A, Yagovkina M, Kirilenko D, Sokolov V, et al. Structure of nanodiamonds prepared by laser synthesis. Phys Solid State. 2013;55(8):1747–53.
Tsukruk VV, Gorbunov VV, Huang Z, Chizhik SA. Dynamic microprobing of viscoelastic polymer properties. Polym Int. 2000;49(5):441–4.
Haghi M, Traini D, Oliver B, Wood LG, Young PM, Chrzanowski W. A ‘soft spot’ for drug transport: modulation of cell stiffness using fatty acids and its impact on drug transport in lung model. J Mater Chem B. 2015. doi:10.1039/C4TB01928H.
Jaffar J, Chrzanowski W, Faiz A, Wolters P, Oliver B, Black J. Primary lung fibroblasts from patients with ipf show increased stiffness which may be due to differential production of ecm proteins. In: Jaffar J, Chrzanowski W, Faiz A, Wolters P, Oliver B, Black J, et al., editors. Respirology. hoboken: Wiley; 2014.
D’Sa D, Chan H-K, Chrzanowski W. Predicting physical stability in pressurized metered dose inhalers via dwell and instantaneous force colloidal probe microscopy. Eur J Pharm Biopharm. 2014;88(1):129–35.
Traini D, Young PM, Rogueda P, Price R. The use of AFM and surface energy measurements to investigate drug-canister material interactions in a model pressurized metered dose inhaler formulation. Aerosol Sci Technol. 2006;40(4):227–36.
Michael Y, Snowden MJ, Chowdhry BZ, Ashurst IC, Davies-Cutting CJ, Riley T. Characterisation of the aggregation behaviour in a salmeterol and fluticasone propionate inhalation aerosol system. Int J Pharm. 2001;221(1–2):165–74.
Young PM, Price R, Lewis D, Edge S, Traini D. Under pressure: predicting pressurized metered dose inhaler interactions using the atomic force microscope. J Colloid Interface Sci. 2003;262(1):298–302.
Tiwari D, Goldman D, Dixit S, Malick W, Madan P. Compatibility evaluation of metered-dose inhaler valve elastomers with tetrafluoroethane (P134a), a non-CFC propellant. Drug Dev Ind Pharm. 1998;24(4):345–52.
Smyth HD. The influence of formulation variables on the performance of alternative propellant-driven metered dose inhalers. Adv Drug Deliv Rev. 2003;55(7):807–28.
Groeger JH, Neugebauer H-J, Schulte C. Method for applying a polymer coating to an internal surface of a container. Us Patent, 2012.
He LH, Swain MV. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J Mech Behav Biomed Mater. 2008;1(1):18–29.
Poole D, Brooks A. The arrangement of crystallites in enamel prisms. Arch Oral Biol. 1961;5(1):14-IN5.
Acil Y, Mobasseri AE, Warnke PH, Terheyden H, Wiltfang J, Springer I. Detection of mature collagen in human dental enamel. Calcif Tissue Int. 2005;76(2):121–6.
Eastoe JE. Organic matrix of tooth enamel. Nature. 1960;187(4735):411–2.
Rettler E, Hoeppener S, Sigusch BW, Schubert US. Mapping the mechanical properties of biomaterials on different length scales: depth-sensing indentation and AFM based nanoindentation. J Mater Chem B. 2013;1(22):2789–806.
Acknowledgments
The authors acknowledge Australian Institute for Nanoscale Science and Technology, The University of Sydney for providing funding (AINST Accelerator Scheme). Also authors acknowledge the use of the facilities as well as the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at The University of Sydney. Dipesh Khanal is a recipient of an Australian Leadership Award scholarship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Khanal, D., Dillon, E., Hau, H. et al. Lorentz contact resonance spectroscopy for nanoscale characterisation of structural and mechanical properties of biological, dental and pharmaceutical materials. J Mater Sci: Mater Med 26, 272 (2015). https://doi.org/10.1007/s10856-015-5605-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5605-1