Abstract
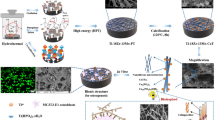
The β-titanium alloy is thought to be a promising alloy using as orthopedic or dental implants owing to its characteristics, which contains low elastic modulus, high corrosion resistance and well biocompatibility. Our previous study has reported that a new β-titanium alloy Ti35Nb2Ta3Zr showed low modulus close to human bone, equal tissue compatibility to a traditional implant alloy Ti6Al4V. In this study, micro-arc oxidation (MAO) was applied on the Ti35Nb2Ta3Zr alloy to enhance its surface characteristics and biocompatibility and osseointegration ability. Two different coatings were formed, TiO2 doped with calcium–phosphate coating (Ca–P) and calcium–phosphate–strontium coating (Ca–P–Sr). Then we evaluated the effects of the MAO coatings on the Ti35Nb2Ta3Zr alloy through in vitro and in vivo tests. As to the characteristics of the coatings, the morphology, chemical composition, surface roughness and contact angle of MAO coatings were tested by scanning electron microscopy, energy dispersive spectroscopy, atomic force microscopy, and video contact-angle measurement system respectively. Besides, we performed MTT assay, ALP test and cell morphology-adhesion test on materials to evaluate the MAOed coating materials’ biocompatibility in vitro. The in vivo experiment was performed through rabbit model. Alloys were implanted into rabbits’ femur shafts, then we performed micro-CT, histological and sequential fluorescent labeling analysis to evaluate implants’ osseointegration ability in vivo. Finally, the Ca–P specimens and Ca–P–Sr specimens exhibited a significant enhancement in surface roughness, hydrophilicity, cell proliferation, cell adhesion. More new bone was found around the Ca–P–Sr coated alloy than Ca–P coated alloy and Ti35Nb2Ta3Zr alloy. In conclusion, the MAO treatment improved in vitro and in vivo performance of Ti35Nb2Ta3Zr alloy. The Ca–P–Sr coating may be a promising modified surface formed by MAO for the novel β-titanium alloy Ti35Nb2Ta3Zr.









Similar content being viewed by others
References
Hao YL, Li SJ, Sun BB, Sui ML, Yang R. Ductile titanium alloy with low Poisson’s ratio. Phys Rev Lett. 2007;98(21):216405.
Wang K. The use of titanium for medical applications in the USA. Mater Sci Eng A. 1996;213(1):134–7.
Snyder SM, Schneider E. Estimation of mechanical properties of cortical bone by computed tomography. J Orthop Res. 1991;9(3):422–31. doi:10.1002/jor.1100090315.
Gefen A. Computational simulations of stress shielding and bone resorption around existing and computer-designed orthopaedic screws. Med Biol Eng Comput. 2002;40(3):311–22.
Rehder D. Vanadium. its role for humans. Metal ions in life sciences. 2013;13:139–69. doi:10.1007/978-94-007-7500-8_5.
Goyer RA, Clarkson TW. Toxic effects of metals. In: Klaassen CD, editors. Casarett & Doull’s toxicology the basic science of poisons, 5th ed. New York: McGraw-Hill Health Professions Division; 1996. ISBN 71054766.
Long M, Rack HJ. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19(18):1621–39.
Guo Y, Chen D, Lu W, Jia Y, Wang L, Zhang X. Corrosion resistance and in vitro response of a novel Ti35Nb2Ta3Zr alloy with a low Young’s modulus. Biomed Mater. 2013;8(5):055004. doi:10.1088/1748-6041/8/5/055004.
Guo Y, Chen D, Cheng M, Lu W, Wang L, Zhang X. The bone tissue compatibility of a new Ti35Nb2Ta3Zr alloy with a low Young’s modulus. Int J Mol Med. 2013;31(3):689–97. doi:10.3892/ijmm.2013.1249.
Hu H, Qiao Y, Meng F, Liu X, Ding C. Enhanced apatite-forming ability and cytocompatibility of porous and nanostructured TiO2/CaSiO3 coating on titanium. Colloids Surf B. 2013;101:83–90. doi:10.1016/j.colsurfb.2012.06.021.
Wang G, Lu Z, Liu X, Zhou X, Ding C, Zreiqat H. Nanostructured glass-ceramic coatings for orthopaedic applications. J R Soc Interface. 2011;8(61):1192–203. doi:10.1098/rsif.2010.0680.
Chen XB, Li YC, Du Plessis J, Hodgson PD, Wen C. Influence of calcium ion deposition on apatite-inducing ability of porous titanium for biomedical applications. Acta Biomater. 2009;5(5):1808–20. doi:10.1016/j.actbio.2009.01.015.
Treves C, Martinesi M, Stio M, Gutierrez A, Jimenez JA, Lopez MF. In vitro biocompatibility evaluation of surface-modified titanium alloys. J Biomed Mater Res A. 2010;92(4):1623–34. doi:10.1002/jbm.a.32507.
Heimann RB, Wirth R. Formation and transformation of amorphous calcium phosphates on titanium alloy surfaces during atmospheric plasma spraying and their subsequent in vitro performance. Biomaterials. 2006;27(6):823–31. doi:10.1016/j.biomaterials.2005.06.029.
Xie Y, Liu X, Huang A, Ding C, Chu PK. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials. 2005;26(31):6129–35. doi:10.1016/j.biomaterials.2005.03.032.
Wei D, Zhou Y, Jia D, Wang Y. Characteristic and in vitro bioactivity of a microarc-oxidized TiO(2)-based coating after chemical treatment. Acta Biomater. 2007;3(5):817–27. doi:10.1016/j.actbio.2007.03.001.
Boanini E, Gazzano M, Bigi A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010;6(6):1882–94. doi:10.1016/j.actbio.2009.12.041.
Bracci B, Torricelli P, Panzavolta S, Boanini E, Giardino R, Bigi A. Effect of Mg2+, Sr2+, and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J Inorg Biochem. 2009;103(12):1666–74. doi:10.1016/j.jinorgbio.2009.09.009.
Kuo MC, Yen SK. The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater Sci Eng C. 2002;20(1–2):153–60. doi:10.1016/S0928-4931(02)00026-7.
Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, Tsouderos Y, Delmas PD, Christiansen C. Incorporation and distribution of strontium in bone. Bone. 2001;28(4):446–53. doi:10.1016/S8756-3282(01)00419-7.
Meunier PJ, Roux C, Ortolani S, Diaz-Curiel M, Compston J, Marquis P, Cormier C, Isaia G, Badurski J, Wark JD, Collette J, Reginster JY. Effects of long-term strontium ranelate treatment on vertebral fracture risk in postmenopausal women with osteoporosis. Osteoporos Int. 2009;20(10):1663–73. doi:10.1007/s00198-008-0825-6.
Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42(1):129–38. doi:10.1016/j.bone.2007.08.043.
Peng S, Liu XS, Huang S, Li Z, Pan H, Zhen W, Luk KD, Guo XE, Lu WW. The cross-talk between osteoclasts and osteoblasts in response to strontium treatment: involvement of osteoprotegerin. Bone. 2011;49(6):1290–8. doi:10.1016/j.bone.2011.08.031.
Zhao L, Wang H, Huo K, Zhang X, Wang W, Zhang Y, Wu Z, Chu PK. The osteogenic activity of strontium loaded titania nanotube arrays on titanium substrates. Biomaterials. 2013;34(1):19–29. doi:10.1016/j.biomaterials.2012.09.041.
Fu DL, Jiang QH, He FM, Yang GL, Liu L. Fluorescence microscopic analysis of bone osseointegration of strontium-substituted hydroxyapatite implants. J Zhejiang Univ Sci B. 2012;13(5):364–71. doi:10.1631/jzus.B1100381.
Li Y, Li Q, Zhu S, Luo E, Li J, Feng G, Liao Y, Hu J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials. 2010;31(34):9006–14. doi:10.1016/j.biomaterials.2010.07.112.
Matsunaga K, Murata H. Strontium substitution in bioactive calcium phosphates: a first-principles study. J Phys Chem B. 2009;113(11):3584–9. doi:10.1021/jp808713m.
Zhang W, Wang G, Liu Y, Zhao X, Zou D, Zhu C, Jin Y, Huang Q, Sun J, Liu X, Jiang X, Zreiqat H. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials. 2013;34(13):3184–95. doi:10.1016/j.biomaterials.2013.01.008.
dos Santos A, Araujo JR, Landi SM, Kuznetsov A, Granjeiro JM, de Sena LA, Achete CA. A study of the physical, chemical and biological properties of TiO2 coatings produced by micro-arc oxidation in a Ca–P-based electrolyte. J Mater Sci Mater Med. 2014;25(7):1769–80. doi:10.1007/s10856-014-5207-3.
Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, Koak JY. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25(14):2867–75. doi:10.1016/j.biomaterials.2003.09.048.
Ventre M, Causa F, Netti PA. Determinants of cell-material crosstalk at the interface: towards engineering of cell instructive materials. J R Soc Interface. 2012;9(74):2017–32. doi:10.1098/rsif.2012.0308.
Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11(9):633–43. doi:10.1038/nrm2957.
Vogler EA. Water and the acute biological response to surfaces. J Biomater Sci Polym Ed. 1999;10(10):1015–45.
Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, Dean DD, Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19(23):2219–32.
del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–41. doi:10.1126/science.1162912.
Sethuraman A, Han M, Kane RS, Belfort G. Effect of surface wettability on the adhesion of proteins. Langmuir. 2004;20(18):7779–88. doi:10.1021/la049454q.
Xu LC, Siedlecki CA. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials. 2007;28(22):3273–83. doi:10.1016/j.biomaterials.2007.03.032.
Rausch-fan X, Qu Z, Wieland M, Matejka M, Schedle A. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Dent Mater. 2008;24(1):102–10. doi:10.1016/j.dental.2007.03.001.
Qu Z, Rausch-Fan X, Wieland M, Matejka M, Schedle A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J Biomed Mater Res A. 2007;82(3):658–68. doi:10.1002/jbm.a.31023.
Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implant. 2010;25(1):63–74.
Oliveira AL, Reis RL, Li P. Strontium-substituted apatite coating grown on Ti6Al4V substrate through biomimetic synthesis. J Biomed Mater Res B Appl Biomater. 2007;83(1):258–65. doi:10.1002/jbm.b.30791.
Li Y, Li X, Song G, Chen K, Yin G, Hu J. Effects of strontium ranelate on osseointegration of titanium implant in osteoporotic rats. Clin Oral Implant Res. 2012;23(9):1038–44. doi:10.1111/j.1600-0501.2011.02252.x.
Maimoun L, Brennan TC, Badoud I, Dubois-Ferriere V, Rizzoli R, Ammann P. Strontium ranelate improves implant osseointegration. Bone. 2010;46(5):1436–41. doi:10.1016/j.bone.2010.01.379.
Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol. 2007;74(3):438–47. doi:10.1016/j.bcp.2007.04.020.
Yamaguchi T. The calcium-sensing receptor in bone. J Bone Miner Metab. 2008;26(4):301–11. doi:10.1007/s00774-008-0843-7.
Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA. 2004;101(14):5140–5. doi:10.1073/pnas.0306141101.
Coulombe J, Faure H, Robin B, Ruat M. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem Biophys Res Commun. 2004;323(4):1184–90. doi:10.1016/j.bbrc.2004.08.209.
Brennan TC, Rybchyn MS, Green W, Atwa S, Conigrave AD, Mason RS. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol. 2009;157(7):1291–300. doi:10.1111/j.1476-5381.2009.00305.x.
Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24(1):33–63. doi:10.1146/annurev.immunol.24.021605.090646.
Offermanns V, Andersen OZ, Falkensammer G, Andersen IH, Almtoft KP, Sorensen S, Sillassen M, Jeppesen CS, Rasse M, Foss M, Kloss F. Enhanced osseointegration of endosseous implants by predictable sustained release properties of strontium. J Biomed Mater Res B Appl Biomater. 2014;. doi:10.1002/jbm.b.33279.
Yamaguchi S, Nath S, Matsushita T, Kokubo T. Controlled release of strontium ions from a bioactive Ti metal with a Ca-enriched surface layer. Acta Biomater. 2014;10(5):2282–9. doi:10.1016/j.actbio.2014.01.026.
Saidak Z, Hay E, Marty C, Barbara A, Marie PJ. Strontium ranelate rebalances bone marrow adipogenesis and osteoblastogenesis in senescent osteopenic mice through NFATc/Maf and Wnt signaling. Aging Cell. 2012;11(3):467–74. doi:10.1111/j.1474-9726.2012.00804.x.
Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2(29):29ra30. doi:10.1126/scitranslmed.3000231.
Fromigue O, Hay E, Barbara A, Marie PJ. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem. 2010;285(33):25251–8. doi:10.1074/jbc.M110.110502.
Almeida M, Han L, Martin-Millan M, O’Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282(37):27298–305. doi:10.1074/jbc.M702811200.
Yan J, Sun JF, Chu PK, Han Y, Zhang YM. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. J Biomed Mater Res A. 2013;101(9):2465–80. doi:10.1002/jbm.a.34548.
Schrooten I, Behets GJ, Cabrera WE, Vercauteren SR, Lamberts LV, Verberckmoes SC, Bervoets AJ, Dams G, Goodman WG, De Broe ME, D’Haese PC. Dose-dependent effects of strontium on bone of chronic renal failure rats. Kidney Int. 2003;63(3):927–35. doi:10.1046/j.1523-1755.2003.00809.x.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (Grant Nos. 81271962 and 81171688), The Science and Technology Commission of Shanghai Municipality, China (Grant No. 12jc1407302).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest in this work.
Additional information
Wei Liu and Mengqi Cheng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, W., Cheng, M., Wahafu, T. et al. The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J Mater Sci: Mater Med 26, 203 (2015). https://doi.org/10.1007/s10856-015-5533-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5533-0