Abstract
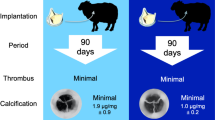
The aim of the study was to estimate the biomechanical properties of heart valves conduit derived from transgenic pigs to determine the usefulness for the preparation of tissue-engineered heart valves. The acellular aortic and pulmonary valve conduits from transgenic pigs were used to estimate the biomechanical properties of the valve. Non-transgenic porcine heart valve conduits were used as a reference. The biomechanics stability of acellular valve conduits decreased both for the transgenic and non-transgenic porcine valves. The energy required to break the native pulmonary valve derived from transgenic pigs was higher (20,475 ± 7,600 J m−2) compared with native non-transgenic pigs (12,140 ± 5,370 J m−2). After acellularization, the energy to break the valves decreased to 14,600 and 8,800 J m−2 for the transgenic pulmonary valve and non-transgenic valve, respectively. The native transgenic pulmonary valve showed a higher extensibility (42.70 %) than the non-transgenic pulmonary valve (35.50 %); the extensibility decreased after acellularization to 41.1 and 31.5 % for the transgenic and non-transgenic valves, respectively. The pulmonary valves derived from transgenic pigs demonstrate better biomechanical properties compared with non-transgenic. Heart valves derived from transgenic pigs can be valuable for the preparation of tissue-engineered bioprostheses, because of their biomechanical properties, stability, reduced immune response, making them safer for clinical applications.








Similar content being viewed by others
References
Rahimtoola SH. Review choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2011;41:893–904.
Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–8.
Schoen FJ. Pathology of heart valve substitution with mechanical and tissue prostheses. In: Silver MD, Gotlieb AI, Schoen FJ, editors. Cardiovascular pathology. New York: Churchill Livingstone; 2001. p. 629–77.
Jamieson WR, von Lipinski O, Miyagishima RT, Burr LH, Janusz MT, Ling H, Fradet GJ, Chan F, Germann E. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after mitral valve replacement. J Thorac Cardiovasc Surg. 2005;129:1301–8.
Burdon TA, Miller DC, Oyer PE, Mitchell RS, Stinson EB, Starnes VA, Shumway NE. Durability of porcine valves at fifteen years in a representative North American patient population. J Thorac Cardiovasc Surg. 1992;103:238–51.
Vongpatanasin W, Hillis LD, Lange RA. Review prosthetic heart valves. N Engl J Med. 1996;335:407–16.
Cascalho M, Platt JL. The immunological barrier to xenotransplantation. Immunity. 2001;14:437–46.
Cooper DK, Koren E, Oriol R. Oligosaccharides and discordant xenotransplantation. Immunol Rev. 1994;141:31–58.
Galili U. Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–2.
Sandrin MS, McKenzie IF. Galα (1,3) Gal, the major xenoantigen(s) recognized in pigs by human natural antibodies. Immunol Rev. 1994;141:169–90.
Golomb G, Schoen FJ, Smith MS, Linden J, Dixon M, Levy RJ. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol. 1987;127:122–30.
Milano A, Bortolotti U, Talenti E, Valfre C, Arbustini E, Valente M. Calcific degeneration as the main cause of porcine bioprosthetic valve dysfunction. Am J Cardiol. 1984;53:1066–70.
Schoen FJ, Levy RJ. Bioprosthetic heart valve failure: pathology and pathogenesis. Cardiol Clin. 1984;2:717–39.
Wilczek P. Heart valve bioprothesis; effect of different acellularizations methods on the biomechanical and morphological properties of porcine aortic and pulmonary valve. Bull Pol Acad Sci. 2010;58:337–42.
Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MKJ, Seebach JD. Lack of galactose-α-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol. 2004;172:6460C–6467.
Lam TT, Hausen B, Boeke-Purkis K, Paniagua R, Lau M, Hook L, Berry G, Higgins J, Duthaler RO, Katopodis AG, Robbins R, Reitz B, Borie D, Schuurman H-J, Morris RE. Hyperacute rejection of hDAF-transgenic pig organ xenografts in cynomolgus monkeys: influence of pre-existing anti-pig antibodies and prevention by the alpha GAL glycoconjugate GAS914. Xenotransplantation. 2004;11:517–24.
Hoerstrup SP, Sodian R, Daebritz S, Wang J, Bacha EA, Martin DP, Moran AM, Guleserian KJ, Sperling JS, Kaushal S, Vacanti JP, Schoen FJ, Mayer JE Jr. Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102:44–9.
Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress and challenges. Ann Biomed Eng. 2006;34:1799–819.
Rieder E, Seebacher G, Kasimir MT, Eichmair E, Winter B, Dekan B, Wolner E, Simon P, Weigel G. Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792–7.
Gonçalves AC, Griffiths LG, Anthony RV, Orton EC. Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J Heart Valve Dis. 2005;14:212–7.
Rieder E, Kasimir M-T, Silberhumer G, Seebacher G, Wolner E, Simon P, Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127:399–405.
Kasimir M-T, Rieder E, Seebacher G, Nigisch A, Dekan B, Wolner E, Weigel G, Simon P. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis. 2006;15:278–86.
Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder E, Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. 2003;23:1002–6.
Bader A, Steinhoff G, Strobl K, Schilling T, Brandes G, Mertsching H, Tsikas D, Froelich J, Haverich A. Engineering of human vascular tissue based on a xenogeneic starter matrix. Transplantation. 2000;70:7–14.
Livi U, Abdulla A-K, Parker R, Olsen EJ, Ross DN. Viability andmorphology of aortic and pulmonary homographs. J Thorac Cardiovasc Surg. 1987;93:755–60.
Gerosa G, Ross DN, Bruecke P, Dziatkowiak A, Mohammed S, Norman D, Davies J, Sbarbati A, Casarotto D. Aortic valve replacement with pulmonary homografts early experience. J Thorac Cardiovasc Surg. 1994;107:424–37.
Liao J, Erinn EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–74.
Li W, Liu W-Y, Yi D-H, Yu S-Q, Jin Z-H. Histological/biological characterization of decellularized bovine jugular vein. Asian Cardiovasc Thorac Ann. 2007;15:91–6.
Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger-de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566–71.
Lee TC, Midura RJ, Hascall VC, Vesely I. The effect of elastin damage on the mechanics of the aortic valve. J Biomech. 2001;34:203–10.
Talman EA, Boughner DR. Glutaraldehydefifixatio alters the internal shear properties of porcine aortic heart valve tissue. Ann Thorac Surg. 1995;60:S369–73.
Fitzpatrick JC, Clark PM, Capaldi FM. Effect of decellularization protocol on the mechanical behavior of porcine descending aorta. Int J Biomater. 2010. doi:10.1155/2010/620503.
Stamm C, Khosravi A, Grabow N, Schmohl K, Treckmann N, Drechsel A, Nan M, Schmitz KP, Axel H, Steinhoff G. Biomatrix/polymer composite material for heart valve tissue engineering. Ann Thorac Surg. 2004;78:2084–93.
Leyh RG, Wilhelmi M, Rebe P, Fischer S, Kofidis T, Haverich A, Mertsching H. In vivo repopulation of xenogeneic and allogeneic acellular valve matrix conduits in the pulmonary circulation. Ann Thorac Surg. 2003;75:1457–63.
Acknowledgments
This work was supported by Grant Applied Research Programme of the National Centre for Research and Development NR 13 0075 06.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wilczek, P., Lesiak, A., Niemiec-Cyganek, A. et al. Biomechanical properties of hybrid heart valve prosthesis utilizing the pigs that do not express the galactose-α-1,3-galactose (α-Gal) antigen derived tissue and tissue engineering technique. J Mater Sci: Mater Med 26, 4 (2015). https://doi.org/10.1007/s10856-014-5329-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-014-5329-7